| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:54:12 UTC |
|---|
| Update Date | 2016-11-09 01:20:50 UTC |
|---|
| Accession Number | CHEM033059 |
|---|
| Identification |
|---|
| Common Name | (all-E)-1,8,10-Heptadecatriene-4,6-diyne-3,12-diol |
|---|
| Class | Small Molecule |
|---|
| Description | (all-E)-1,8,10-Heptadecatriene-4,6-diyne-3,12-diol is found in tea. (all-E)-1,8,10-Heptadecatriene-4,6-diyne-3,12-diol is a constituent of ginseng. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
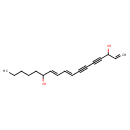
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C17H22O2 |
|---|
| Average Molecular Mass | 258.355 g/mol |
|---|
| Monoisotopic Mass | 258.162 g/mol |
|---|
| CAS Registry Number | 143966-10-3 |
|---|
| IUPAC Name | (8E,10E)-heptadeca-1,8,10-trien-4,6-diyne-3,12-diol |
|---|
| Traditional Name | (8E,10E)-heptadeca-1,8,10-trien-4,6-diyne-3,12-diol |
|---|
| SMILES | CCCCCC(O)\C=C\C=C\C#CC#CC(O)C=C |
|---|
| InChI Identifier | InChI=1S/C17H22O2/c1-3-5-10-14-17(19)15-12-9-7-6-8-11-13-16(18)4-2/h4,7,9,12,15-19H,2-3,5,10,14H2,1H3/b9-7+,15-12+ |
|---|
| InChI Key | ADJNQGJVAJRCCO-KDFHGORWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as long-chain fatty alcohols. These are fatty alcohols that have an aliphatic tail of 13 to 21 carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty alcohols |
|---|
| Direct Parent | Long-chain fatty alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Long chain fatty alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05n0-4920000000-133bcc755a20a10db1f8 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-002r-9018000000-63af16d0923f2a5ce1c6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052f-0190000000-9ec809d49f798fb7d61b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0abc-9860000000-4ba887722176bdb396dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ki7-9200000000-55699ff1bc79f0d60b84 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0090000000-fcc1813aebe462949803 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-2290000000-d4619ccd7a4d4f6a7e2a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pi4-9720000000-e38aad2b3180d1a0fa0c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0590000000-c09f290d0b68c973aeee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-022c-1790000000-1226779d3cac29c56855 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f9b-4910000000-8fcc4664b274b44ae43a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4r-0090000000-f2fc768c5e0b599ea6a6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0690000000-cd31cbcd0ea011b3d050 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00sj-4910000000-6e4ac3f05be598846468 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039737 |
|---|
| FooDB ID | FDB019379 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014868 |
|---|
| ChEBI ID | 173847 |
|---|
| PubChem Compound ID | 131752714 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|