| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:54:04 UTC |
|---|
| Update Date | 2016-11-09 01:20:50 UTC |
|---|
| Accession Number | CHEM033056 |
|---|
| Identification |
|---|
| Common Name | 2,3,5,7,9-Pentathiadecane 2,2-dioxide |
|---|
| Class | Small Molecule |
|---|
| Description | 2,3,5,7,9-Pentathiadecane 2,2-dioxide is found in mushrooms. 2,3,5,7,9-Pentathiadecane 2,2-dioxide is isolated from the shiitake mushroom (Lentinus edodes). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
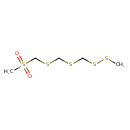
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| SE-3 | HMDB | | Methanesulphonyl[({[(methyldisulphanyl)methyl]sulphanyl}methyl)sulphanyl]methane | Generator |
|
|---|
| Chemical Formula | C5H12O2S5 |
|---|
| Average Molecular Mass | 264.473 g/mol |
|---|
| Monoisotopic Mass | 263.944 g/mol |
|---|
| CAS Registry Number | 156430-94-3 |
|---|
| IUPAC Name | methanesulfonyl[({[(methyldisulfanyl)methyl]sulfanyl}methyl)sulfanyl]methane |
|---|
| Traditional Name | methanesulfonyl[({[(methyldisulfanyl)methyl]sulfanyl}methyl)sulfanyl]methane |
|---|
| SMILES | CSSCSCSCS(C)(=O)=O |
|---|
| InChI Identifier | InChI=1S/C5H12O2S5/c1-8-11-4-9-3-10-5-12(2,6)7/h3-5H2,1-2H3 |
|---|
| InChI Key | BHWJQDRVDKQMFE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sulfones. Sulfones are compounds containing a sulfonyl group( which as the general structure RS(=O)2R' (R,R' =alkyl, aryl)) attached to two carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organosulfur compounds |
|---|
| Class | Sulfonyls |
|---|
| Sub Class | Sulfones |
|---|
| Direct Parent | Sulfones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sulfone
- Thioacetal
- Dialkyldisulfide
- Organic disulfide
- Dialkylthioether
- Sulfenyl compound
- Thioether
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000f-8900000000-b89e41a89ee49a4d20e7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0190000000-df7e74fb6facf530a3e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-3910000000-e9dc5f2e4ddfe51dc2c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00dj-8920000000-0360c0f89361dabe5a3e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1290000000-ed60b08e6727bab4c7be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9230000000-a94453402e99db6d9def | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9400000000-bff6f18d188d9a1067e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-9200000000-3c66822f01a5a6694991 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9200000000-2f90fda370a6b1c93e92 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-3d8d3ff7b3f347027ed5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03fr-2950000000-6fb8b82473b57440fbe8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002o-9200000000-a4e3acf39724c9639c54 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0005-9000000000-c0d948d5376efcb94e2e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039734 |
|---|
| FooDB ID | FDB019376 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00057115 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9952282 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 11777600 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|