| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:52:20 UTC |
|---|
| Update Date | 2016-11-09 01:20:50 UTC |
|---|
| Accession Number | CHEM033022 |
|---|
| Identification |
|---|
| Common Name | Oryzalexin E |
|---|
| Class | Small Molecule |
|---|
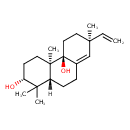
| Description | A tricyclic diterpenoid that is ent-sandaracopimaradiene bearing two additional hydroxy substituents at the 3alpha- and 9beta-positions. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3alpha,9beta-Dihydroxy-ent-sandaracopimaradiene | ChEBI | | ent-Sandaracopimaradien-3alpha,7beta-diol | ChEBI | | 3a,9b-Dihydroxy-ent-sandaracopimaradiene | Generator | | 3Α,9β-dihydroxy-ent-sandaracopimaradiene | Generator | | ent-Sandaracopimaradien-3a,7b-diol | Generator | | ent-Sandaracopimaradien-3α,7β-diol | Generator |
|
|---|
| Chemical Formula | C20H32O2 |
|---|
| Average Molecular Mass | 304.467 g/mol |
|---|
| Monoisotopic Mass | 304.240 g/mol |
|---|
| CAS Registry Number | 150943-96-7 |
|---|
| IUPAC Name | (2R,4aR,4bS,7S,10aR)-7-ethenyl-1,1,4a,7-tetramethyl-1,2,3,4,4a,4b,5,6,7,9,10,10a-dodecahydrophenanthrene-2,4b-diol |
|---|
| Traditional Name | (2R,4aR,4bS,7S,10aR)-7-ethenyl-1,1,4a,7-tetramethyl-2,3,4,5,6,9,10,10a-octahydrophenanthrene-2,4b-diol |
|---|
| SMILES | [H][C@]12CCC3=C[C@@](C)(CC[C@@]3(O)[C@]1(C)CC[C@@H](O)C2(C)C)C=C |
|---|
| InChI Identifier | InChI=1S/C20H32O2/c1-6-18(4)11-12-20(22)14(13-18)7-8-15-17(2,3)16(21)9-10-19(15,20)5/h6,13,15-16,21-22H,1,7-12H2,2-5H3/t15-,16-,18-,19-,20+/m1/s1 |
|---|
| InChI Key | RGLTYROISYBKIW-BDUQCRIQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydrophenanthrenes. These are a phenanthrene derivative where at least one ring CC bond is substituted by hydrogenation. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenanthrenes and derivatives |
|---|
| Sub Class | Hydrophenanthrenes |
|---|
| Direct Parent | Hydrophenanthrenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydrophenanthrene
- Tertiary alcohol
- Cyclic alcohol
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002r-0690000000-f5370294d497f51a221f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-003r-7053900000-647da576f4de0583fcc2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0092000000-24eb84409ea59729c425 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05n0-6291000000-b1aa0995efc8649a656f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-9130000000-d6c3dd4714c155fd19bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0049000000-adca20b041c892561103 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udr-0097000000-1e9e9416d4f667152ee0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fe0-2090000000-b963004147828af19ec7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-f9d10b76ade80bd401ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0019000000-97dc9de2ef2dab255fc5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-1029000000-02a4e499444e8891ce43 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ap0-0093000000-007a2a1ef25dcc9f8415 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0692000000-362a90923765bfcd7c43 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-5790000000-4f838ce109643df337ff | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039702 |
|---|
| FooDB ID | FDB019331 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00034093 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-16611 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30777361 |
|---|
| ChEBI ID | 78259 |
|---|
| PubChem Compound ID | 86289490 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|