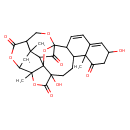| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:52:10 UTC |
|---|
| Update Date | 2016-11-09 01:20:50 UTC |
|---|
| Accession Number | CHEM033018 |
|---|
| Identification |
|---|
| Common Name | Isophysalin G |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Physalis alkekengi (winter cherry). Isophysalin G is found in fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6-((4-Amino-6-quinazolinyl)thio)-4-quinazolinylamine | HMDB |
|
|---|
| Chemical Formula | C28H30O10 |
|---|
| Average Molecular Mass | 526.532 g/mol |
|---|
| Monoisotopic Mass | 526.184 g/mol |
|---|
| CAS Registry Number | 152221-21-1 |
|---|
| IUPAC Name | 5,12-dihydroxy-2,9,26-trimethyl-3,19,23,28-tetraoxaoctacyclo[16.9.1.1¹⁸,²⁷.0¹,⁵.0²,²⁴.0⁸,¹⁷.0⁹,¹⁴.0²¹,²⁶]nonacosa-13,15-diene-4,10,22,29-tetrone |
|---|
| Traditional Name | 5,12-dihydroxy-2,9,26-trimethyl-3,19,23,28-tetraoxaoctacyclo[16.9.1.1¹⁸,²⁷.0¹,⁵.0²,²⁴.0⁸,¹⁷.0⁹,¹⁴.0²¹,²⁶]nonacosa-13,15-diene-4,10,22,29-tetrone |
|---|
| SMILES | CC12OC(=O)C3(O)CCC4C(C=CC5=CC(O)CC(=O)C45C)C45OC13C(C4=O)C1(C)CC2OC(=O)C1CO5 |
|---|
| InChI Identifier | InChI=1S/C28H30O10/c1-23-10-18-25(3)28-19(23)20(31)27(38-28,35-11-16(23)21(32)36-18)15-5-4-12-8-13(29)9-17(30)24(12,2)14(15)6-7-26(28,34)22(33)37-25/h4-5,8,13-16,18-19,29,34H,6-7,9-11H2,1-3H3 |
|---|
| InChI Key | JMNMXSXLYDOMTI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as physalins and derivatives. These are steroidal constituents of Physalis plants which possess an unusual 13,14-seco-16,24-cyclo-steroidal ring skeleton (where the bond that is normally present between the 13 and 14 positions in other steroids is broken while a new bond between positions 16 and 24 is formed). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Physalins and derivatives |
|---|
| Direct Parent | Physalins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Physalin skeleton
- Delta_valerolactone
- Ketal
- Oxepane
- Cyclohexenone
- Delta valerolactone
- Gamma butyrolactone
- Oxane
- Dicarboxylic acid or derivatives
- 3-furanone
- Cyclic alcohol
- Tertiary alcohol
- Tetrahydrofuran
- Lactone
- Carboxylic acid ester
- Secondary alcohol
- Ketone
- Acetal
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Organic oxide
- Organic oxygen compound
- Alcohol
- Hydrocarbon derivative
- Carbonyl group
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-0900100000-1161fc126d8ce46ed8ff | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-03di-0090001000-c1b6d8dd4b21fb312f47 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-0000290000-880101d4bc004b3925ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-0101970000-dd2356c2de680e091d61 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k96-5901500000-a8dd52ad366e1cb80890 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000390000-e4fd65c57b71ace4d61e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-057i-0000790000-2ce1af94be8335e4f35b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f79-0750900000-4c38d17f187c1078b362 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0000090000-18aac04a6d9922073eca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0200960000-72fbc4007f3258871a78 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zi3-0600900000-1deebf17db6be753c2a8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000090000-a09e301130798fa02997 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-056r-0000390000-fca6de4784905af47efe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0k92-0201930000-b341187949bbe7594756 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039698 |
|---|
| FooDB ID | FDB019327 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 74336861 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|