| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:52:08 UTC |
|---|
| Update Date | 2016-11-09 01:20:50 UTC |
|---|
| Accession Number | CHEM033017 |
|---|
| Identification |
|---|
| Common Name | Isophysalin B |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Physalis alkekengi (winter cherry). Isophysalin B is found in fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
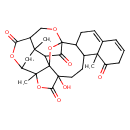
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C28H30O9 |
|---|
| Average Molecular Mass | 510.532 g/mol |
|---|
| Monoisotopic Mass | 510.189 g/mol |
|---|
| CAS Registry Number | 26435-77-8 |
|---|
| IUPAC Name | 5-hydroxy-2,9,26-trimethyl-3,19,23,28-tetraoxaoctacyclo[16.9.1.1¹⁸,²⁷.0¹,⁵.0²,²⁴.0⁸,¹⁷.0⁹,¹⁴.0²¹,²⁶]nonacosa-12,14-diene-4,10,22,29-tetrone |
|---|
| Traditional Name | 5-hydroxy-2,9,26-trimethyl-3,19,23,28-tetraoxaoctacyclo[16.9.1.1¹⁸,²⁷.0¹,⁵.0²,²⁴.0⁸,¹⁷.0⁹,¹⁴.0²¹,²⁶]nonacosa-12,14-diene-4,10,22,29-tetrone |
|---|
| SMILES | CC12OC(=O)C3(O)CCC4C(CC=C5C=CCC(=O)C45C)C45OC13C(C4=O)C1(C)CC2OC(=O)C1CO5 |
|---|
| InChI Identifier | InChI=1S/C28H30O9/c1-23-11-18-25(3)28-19(23)20(30)27(37-28,34-12-16(23)21(31)35-18)15-8-7-13-5-4-6-17(29)24(13,2)14(15)9-10-26(28,33)22(32)36-25/h4-5,7,14-16,18-19,33H,6,8-12H2,1-3H3 |
|---|
| InChI Key | HRUAQJXBUYEBPK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as physalins and derivatives. These are steroidal constituents of Physalis plants which possess an unusual 13,14-seco-16,24-cyclo-steroidal ring skeleton (where the bond that is normally present between the 13 and 14 positions in other steroids is broken while a new bond between positions 16 and 24 is formed). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Physalins and derivatives |
|---|
| Direct Parent | Physalins and derivatives |
|---|
| Alternative Parents | Not Available |
|---|
| Substituents | Not Available |
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-0900100000-42d739d100c2d48023fd | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dr-1900030000-c14d5121bafd85f21afc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000690000-60570ba5a6edb1fce68d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ox-0100920000-6dabfd9f8a920691fdff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ktf-8900400000-c1d73a6289cb197cf8ed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000490000-a90e0824e2b75b396ee1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0aor-0000940000-a326db048283dfb4d4f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ds-1910600000-743724e8dd993c3937c6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000190000-dfe95957edd518c62d6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03ec-0501950000-e54c3e8f7dc71ff0b64f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4u-2901500000-bd19e22e2de707375ce7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000090000-fba8e660fda6f9e81f56 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0000390000-faec354c6a1217395439 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-069r-0302910000-f283e119feb7e07b1939 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039697 |
|---|
| FooDB ID | FDB019326 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 17262464 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 22748169 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|