| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:49:19 UTC |
|---|
| Update Date | 2016-11-09 01:20:49 UTC |
|---|
| Accession Number | CHEM032946 |
|---|
| Identification |
|---|
| Common Name | 2-Galloylglucose |
|---|
| Class | Small Molecule |
|---|
| Description | 2-Galloylglucose is found in green vegetables. 2-Galloylglucose is a constituent of commercial rhubarb (Rheum species). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
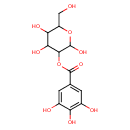
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,4,5-Trihydroxy-6-(hydroxymethyl)oxan-3-yl 3,4,5-trihydroxybenzoic acid | HMDB |
|
|---|
| Chemical Formula | C13H16O10 |
|---|
| Average Molecular Mass | 332.260 g/mol |
|---|
| Monoisotopic Mass | 332.074 g/mol |
|---|
| CAS Registry Number | 98917-85-2 |
|---|
| IUPAC Name | 2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl 3,4,5-trihydroxybenzoate |
|---|
| Traditional Name | 2,4,5-trihydroxy-6-(hydroxymethyl)oxan-3-yl 3,4,5-trihydroxybenzoate |
|---|
| SMILES | OCC1OC(O)C(OC(=O)C2=CC(O)=C(O)C(O)=C2)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C13H16O10/c14-3-7-9(18)10(19)11(13(21)22-7)23-12(20)4-1-5(15)8(17)6(16)2-4/h1-2,7,9-11,13-19,21H,3H2 |
|---|
| InChI Key | DZAITDYEHYKPKZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as galloyl esters. These are organic compounds that contain an ester derivative of 3,4,5-trihydroxybenzoic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Galloyl esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Galloyl ester
- Hexose monosaccharide
- P-hydroxybenzoic acid alkyl ester
- M-hydroxybenzoic acid ester
- P-hydroxybenzoic acid ester
- Benzoate ester
- Pyrogallol derivative
- Benzenetriol
- Benzoyl
- Phenol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Monosaccharide
- Oxane
- Secondary alcohol
- Carboxylic acid ester
- Hemiacetal
- Carboxylic acid derivative
- Oxacycle
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Polyol
- Alcohol
- Organic oxygen compound
- Primary alcohol
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0umu-9633000000-aba9a58204300719abc5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-004i-3603009000-ede87035edde20f86c9a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gx0-0906000000-32f49d7f00b158a3bfec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0w29-1902000000-e8e20f426e5a08a0359b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-5900000000-58d4e97b9df583b0cc7a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-02ai-2936000000-05230801f18cfb229bde | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-2901000000-07eefc22e1241df70f74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-016r-3900000000-c1eb95621591d63207fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0549000000-020fb532ba6712c2a75d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0j6r-1932000000-8d0cb77b4468ad389de0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-07fr-6910000000-d0872bb701cdb590a0a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-0269000000-11b52a24d5e7e202ba7d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-0695000000-7af19509f99f5f806a4a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ufr-3900000000-ff3018222bfd9c3de0be | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039625 |
|---|
| FooDB ID | FDB019253 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 14034252 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|