| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:37:13 UTC |
|---|
| Update Date | 2016-11-09 01:20:46 UTC |
|---|
| Accession Number | CHEM032684 |
|---|
| Identification |
|---|
| Common Name | Methyl 6-O-galloyl-beta-D-glucopyranoside |
|---|
| Class | Small Molecule |
|---|
| Description | Methyl 6-O-galloyl-beta-D-glucopyranoside is found in green vegetables. Tannin constituent of burnet bloodwort (Sanguisorba officinalis). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
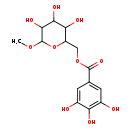
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methyl 6-O-galloyl-b-D-glucopyranoside | Generator | | Methyl 6-O-galloyl-β-D-glucopyranoside | Generator | | 2-fluoro-2,2-Dinitroethyl carbonate(2:1) | HMDB | | Bis(2-fluoro-2,2-bis(hydroxy(oxido)amino)ethyl) carbonate | HMDB | | (3,4,5-Trihydroxy-6-methoxyoxan-2-yl)methyl 3,4,5-trihydroxybenzoic acid | Generator |
|
|---|
| Chemical Formula | C14H18O10 |
|---|
| Average Molecular Mass | 346.287 g/mol |
|---|
| Monoisotopic Mass | 346.090 g/mol |
|---|
| CAS Registry Number | 88647-06-7 |
|---|
| IUPAC Name | (3,4,5-trihydroxy-6-methoxyoxan-2-yl)methyl 3,4,5-trihydroxybenzoate |
|---|
| Traditional Name | (3,4,5-trihydroxy-6-methoxyoxan-2-yl)methyl 3,4,5-trihydroxybenzoate |
|---|
| SMILES | COC1OC(COC(=O)C2=CC(O)=C(O)C(O)=C2)C(O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C14H18O10/c1-22-14-12(20)11(19)10(18)8(24-14)4-23-13(21)5-2-6(15)9(17)7(16)3-5/h2-3,8,10-12,14-20H,4H2,1H3 |
|---|
| InChI Key | FNCIIGZVDLRIDE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as galloyl esters. These are organic compounds that contain an ester derivative of 3,4,5-trihydroxybenzoic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Galloyl esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Galloyl ester
- P-hydroxybenzoic acid alkyl ester
- M-hydroxybenzoic acid ester
- P-hydroxybenzoic acid ester
- Glycosyl compound
- O-glycosyl compound
- Benzoate ester
- Benzenetriol
- Pyrogallol derivative
- Benzoyl
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monosaccharide
- Oxane
- Carboxylic acid ester
- Secondary alcohol
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Oxacycle
- Acetal
- Carboxylic acid derivative
- Polyol
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ufr-3923000000-30c173ff3930fcf829c3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-01b9-5345019000-410c581c583002d6374b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0907000000-b4d926b654ff2202bcd6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fb9-0902000000-5b739b60f43d691e39e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ufr-2900000000-0a39d7eb2057b4ce3e2f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014j-1915000000-8e04a0a6ff0e62dd8c53 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-016r-1901000000-11939a63e350e5963ecd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-016r-5900000000-a7247d0f5a4c3e6a9efa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0109000000-eedbd623ced2afef0ab8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004j-1923000000-c12a1dac92200b58b694 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05r0-3910000000-12270d991547f00f230f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0309000000-fe74184d9ebc8a86bcce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6t-1973000000-d28cc46410dbb0440a01 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0w29-4920000000-602d9434cb0d77c7cc9e | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039354 |
|---|
| FooDB ID | FDB018910 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 78385296 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|