| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:35:13 UTC |
|---|
| Update Date | 2016-11-09 01:20:45 UTC |
|---|
| Accession Number | CHEM032635 |
|---|
| Identification |
|---|
| Common Name | Epicatechin-(4beta->8)-epigallocatechin 3,3'-digallate |
|---|
| Class | Small Molecule |
|---|
| Description | Epicatechin 3-O-gallate-(4beta->8)-epigallocatechin 3-O-gallate is found in tea. Epicatechin 3-O-gallate-(4beta->8)-epigallocatechin 3-O-gallate is isolated from commercial oolong tea (Camellia sinensis var. viridis). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
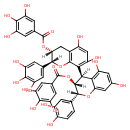
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Epicatechin 3-O-gallate-(4b->8)-epigallocatechin 3-O-gallate | Generator | | Epicatechin 3-O-gallate-(4β->8)-epigallocatechin 3-O-gallate | Generator | | Epicatechin 3-O-gallic acid-(4b->8)-epigallocatechin 3-O-gallic acid | Generator | | Epicatechin 3-O-gallic acid-(4beta->8)-epigallocatechin 3-O-gallic acid | Generator | | Epicatechin 3-O-gallic acid-(4β->8)-epigallocatechin 3-O-gallic acid | Generator | | 3-O-Galloylepicatechin-(4beta->8)-epigallocatechin-3-O-gallate | HMDB | | Epicatechin 3-O-gallate(4b->8)epigallocatechin-3-O-gallate | HMDB | | (2R,3R)-8-[(2R,3R,4R)-2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3-(3,4,5-trihydroxybenzoyloxy)-3,4-dihydro-2H-1-benzopyran-4-yl]-5,7-dihydroxy-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3-yl 3,4,5-trihydroxybenzoic acid | Generator | | Epicatechin-(4b->8)-epigallocatechin 3,3'-digallate | Generator | | Epicatechin-(4b->8)-epigallocatechin 3,3'-digallic acid | Generator | | Epicatechin-(4beta->8)-epigallocatechin 3,3'-digallic acid | Generator | | Epicatechin-(4β->8)-epigallocatechin 3,3'-digallate | Generator | | Epicatechin-(4β->8)-epigallocatechin 3,3'-digallic acid | Generator |
|
|---|
| Chemical Formula | C44H34O21 |
|---|
| Average Molecular Mass | 898.728 g/mol |
|---|
| Monoisotopic Mass | 898.159 g/mol |
|---|
| CAS Registry Number | 126715-90-0 |
|---|
| IUPAC Name | (2R,3R,4R)-4-[(2R,3R)-5,7-dihydroxy-3-(3,4,5-trihydroxybenzoyloxy)-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-8-yl]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-1-benzopyran-3-yl 3,4,5-trihydroxybenzoate |
|---|
| Traditional Name | (2R,3R,4R)-4-[(2R,3R)-5,7-dihydroxy-3-(3,4,5-trihydroxybenzoyloxy)-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-8-yl]-2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3,4-dihydro-2H-1-benzopyran-3-yl 3,4,5-trihydroxybenzoate |
|---|
| SMILES | OC1=CC2=C([C@@H]([C@@H](OC(=O)C3=CC(O)=C(O)C(O)=C3)[C@H](O2)C2=CC=C(O)C(O)=C2)C2=C(O)C=C(O)C3=C2O[C@@H]([C@@H](C3)OC(=O)C2=CC(O)=C(O)C(O)=C2)C2=CC(O)=C(O)C(O)=C2)C(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C44H34O21/c45-18-10-23(49)33-31(11-18)62-40(14-1-2-20(46)22(48)3-14)42(65-44(61)17-8-29(55)38(59)30(56)9-17)35(33)34-24(50)13-21(47)19-12-32(63-43(60)16-6-27(53)37(58)28(54)7-16)39(64-41(19)34)15-4-25(51)36(57)26(52)5-15/h1-11,13,32,35,39-40,42,45-59H,12H2/t32-,35-,39-,40-,42-/m1/s1 |
|---|
| InChI Key | FUNWNVWJOMCWIL-WZPNJOEPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthones. These are polycyclic aromatic compounds containing a xanthene moiety conjugated to a ketone group at carbon 9. Xanthene is a tricyclic compound made up of two benzene rings linearly fused to each other through a pyran ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzopyrans |
|---|
| Sub Class | 1-benzopyrans |
|---|
| Direct Parent | Xanthones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthone
- Phenolic glycoside
- Galloyl ester
- Gallic acid or derivatives
- P-hydroxybenzoic acid alkyl ester
- Chromone
- M-hydroxybenzoic acid ester
- P-hydroxybenzoic acid ester
- C-glycosyl compound
- Glycosyl compound
- Benzoate ester
- Pyrogallol derivative
- Benzoic acid or derivatives
- Benzenetriol
- Benzoyl
- Phenol
- 1-hydroxy-4-unsubstituted benzenoid
- Pyranone
- 1-hydroxy-2-unsubstituted benzenoid
- Pyran
- Benzenoid
- Oxane
- Monocyclic benzene moiety
- Monosaccharide
- Heteroaromatic compound
- Vinylogous acid
- Secondary alcohol
- Carboxylic acid ester
- Polyol
- Ether
- Dialkyl ether
- Carboxylic acid derivative
- Oxacycle
- Monocarboxylic acid or derivatives
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004j-0510190450-c8b3cb9cb2be88893f02 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zfu-0950341110-19781e50cd07b03c29db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfr-0980110000-30004be43717cada09dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0300100390-c0d7cba70e9df31ef305 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-016r-0914300430-e43544d33d91d817390b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-016r-0900000100-b261b6863f1f519616f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000000890-b864c0861b6ed55485a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004j-0400000890-beb2f1085074c959ed9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02bu-0401000190-975205984f3149c58eff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00c1-0100002950-9d3aa37ffcff3c16293e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0fbd-0200120980-f43bfe178e976ef6e2b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0035-0300220690-42ddaef32deaea45ba76 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039304 |
|---|
| FooDB ID | FDB018850 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00009219 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9796831 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 11622083 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|