| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:34:17 UTC |
|---|
| Update Date | 2016-11-09 01:20:45 UTC |
|---|
| Accession Number | CHEM032614 |
|---|
| Identification |
|---|
| Common Name | 2-O-Galloylsucrose |
|---|
| Class | Small Molecule |
|---|
| Description | 2-O-Galloylsucrose is found in green vegetables. 2-O-Galloylsucrose is isolated from the commercial Chinese rhubarb (Rheum species). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
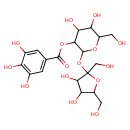
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-{[3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl 3,4,5-trihydroxybenzoic acid | HMDB |
|
|---|
| Chemical Formula | C19H26O15 |
|---|
| Average Molecular Mass | 494.401 g/mol |
|---|
| Monoisotopic Mass | 494.127 g/mol |
|---|
| CAS Registry Number | 115713-46-7 |
|---|
| IUPAC Name | 2-{[3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl 3,4,5-trihydroxybenzoate |
|---|
| Traditional Name | 2-{[3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}-4,5-dihydroxy-6-(hydroxymethyl)oxan-3-yl 3,4,5-trihydroxybenzoate |
|---|
| SMILES | OCC1OC(CO)(OC2OC(CO)C(O)C(O)C2OC(=O)C2=CC(O)=C(O)C(O)=C2)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C19H26O15/c20-3-9-12(26)14(28)15(32-17(30)6-1-7(23)11(25)8(24)2-6)18(31-9)34-19(5-22)16(29)13(27)10(4-21)33-19/h1-2,9-10,12-16,18,20-29H,3-5H2 |
|---|
| InChI Key | LNFBBISCIPAUAE-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as galloyl esters. These are organic compounds that contain an ester derivative of 3,4,5-trihydroxybenzoic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Galloyl esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Galloyl ester
- P-hydroxybenzoic acid alkyl ester
- M-hydroxybenzoic acid ester
- P-hydroxybenzoic acid ester
- O-glycosyl compound
- Glycosyl compound
- Disaccharide
- C-glycosyl compound
- Benzoate ester
- Benzenetriol
- Pyrogallol derivative
- Benzoyl
- Ketal
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Oxane
- Tetrahydrofuran
- Secondary alcohol
- Carboxylic acid ester
- Acetal
- Oxacycle
- Carboxylic acid derivative
- Organoheterocyclic compound
- Polyol
- Monocarboxylic acid or derivatives
- Alcohol
- Organic oxygen compound
- Primary alcohol
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0nor-9502800000-1b299890c242817d7de1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0fis-6901114000-3e5fca379d304f860570 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0900000000-46dec674dc02fc796de9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03yi-0903000000-0230ab077b985dcbf5e0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03du-9700000000-c40b73493504ed75b1d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1901100000-fc7a5e12fcb68662e303 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fr-0900000000-2dddffd05d280f01f129 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00mn-4900000000-b8250999368a2a8a8e42 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01ox-0705900000-23521783077106ad2dd5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-2603900000-9ce98969c96afaebb034 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-5901100000-3c15da46441408e0afd3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0202900000-957ae3c7603c36beea12 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-003s-1903300000-4d628ed688832a834618 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f6w-7902000000-679cad0d9fa8db30aa20 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039281 |
|---|
| FooDB ID | FDB018826 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00056524 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 14055563 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|