| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:32:32 UTC |
|---|
| Update Date | 2016-11-09 01:20:45 UTC |
|---|
| Accession Number | CHEM032588 |
|---|
| Identification |
|---|
| Common Name | Sanguiin H8 |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
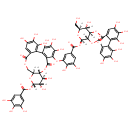
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (10R,11S,12S,13S,15S)-6-[5-({[(10S,11R,13S,14S,15R)-3,4,5,14,20,21,22-heptahydroxy-13-(hydroxymethyl)-8,17-dioxo-9,12,16-trioxatetracyclo[16.4.0.0,.0,]docosa-1(22),2,4,6,18,20-hexaen-11-yl]oxy}carbonyl)-2,3-dihydroxyphenoxy]-3,4,5,11,12,21,22,23-octahydroxy-8,18-dioxo-9,14,17-trioxatetracyclo[17.4.0.0,.0,]tricosa-1(23),2(7),3,5,19,21-hexaen-13-yl 3,4,5-trihydroxybenzoic acid | Generator | | (10R,11S,12S,13S,15S)-6-[5-({[(10S,11R,13S,14S,15R)-3,4,5,14,20,21,22-heptahydroxy-13-(hydroxymethyl)-8,17-dioxo-9,12,16-trioxatetracyclo[16.4.0.0²,⁷.0¹⁰,¹⁵]docosa-1(22),2,4,6,18,20-hexaen-11-yl]oxy}carbonyl)-2,3-dihydroxyphenoxy]-3,4,5,11,12,21,22,23-octahydroxy-8,18-dioxo-9,14,17-trioxatetracyclo[17.4.0.0²,⁷.0¹⁰,¹⁵]tricosa-1(23),2(7),3,5,19,21-hexaen-13-yl 3,4,5-trihydroxybenzoic acid | Generator |
|
|---|
| Chemical Formula | C54H42O36 |
|---|
| Average Molecular Mass | 1266.894 g/mol |
|---|
| Monoisotopic Mass | 1266.146 g/mol |
|---|
| CAS Registry Number | 98975-04-3 |
|---|
| IUPAC Name | (10R,11S,12S,13S,15S)-6-[5-({[(10S,11R,13S,14S,15R)-3,4,5,14,20,21,22-heptahydroxy-13-(hydroxymethyl)-8,17-dioxo-9,12,16-trioxatetracyclo[16.4.0.0²,⁷.0¹⁰,¹⁵]docosa-1(22),2,4,6,18,20-hexaen-11-yl]oxy}carbonyl)-2,3-dihydroxyphenoxy]-3,4,5,11,12,21,22,23-octahydroxy-8,18-dioxo-9,14,17-trioxatetracyclo[17.4.0.0²,⁷.0¹⁰,¹⁵]tricosa-1(23),2(7),3,5,19,21-hexaen-13-yl 3,4,5-trihydroxybenzoate |
|---|
| Traditional Name | (10R,11S,12S,13S,15S)-6-[5-({[(10S,11R,13S,14S,15R)-3,4,5,14,20,21,22-heptahydroxy-13-(hydroxymethyl)-8,17-dioxo-9,12,16-trioxatetracyclo[16.4.0.0²,⁷.0¹⁰,¹⁵]docosa-1(22),2,4,6,18,20-hexaen-11-yl]oxy}carbonyl)-2,3-dihydroxyphenoxy]-3,4,5,11,12,21,22,23-octahydroxy-8,18-dioxo-9,14,17-trioxatetracyclo[17.4.0.0²,⁷.0¹⁰,¹⁵]tricosa-1(23),2(7),3,5,19,21-hexaen-13-yl 3,4,5-trihydroxybenzoate |
|---|
| SMILES | [H][C@@]1(CO)O[C@]([H])(OC(=O)C2=CC(O)=C(O)C(OC3=C(O)C(O)=C(O)C4=C3C(=O)O[C@@]3([H])[C@]([H])(COC(=O)C5=CC(O)=C(O)C(O)=C45)O[C@@]([H])(OC(=O)C4=CC(O)=C(O)C(O)=C4)[C@@]([H])(O)[C@]3([H])O)=C2)[C@@]2([H])OC(=O)C3=CC(O)=C(O)C(O)=C3C3=C(O)C(O)=C(O)C=C3C(=O)O[C@]2([H])[C@@]1([H])O |
|---|
| InChI Identifier | InChI=1S/C54H42O36/c55-8-22-34(67)45-46(88-51(80)14-7-20(61)32(65)36(69)25(14)24-13(50(79)87-45)6-19(60)31(64)35(24)68)54(84-22)90-48(77)11-3-17(58)30(63)21(4-11)83-44-28-27(38(71)39(72)40(44)73)26-12(5-18(59)33(66)37(26)70)49(78)82-9-23-43(86-52(28)81)41(74)42(75)53(85-23)89-47(76)10-1-15(56)29(62)16(57)2-10/h1-7,22-23,34,41-43,45-46,53-75H,8-9H2/t22-,23-,34-,41-,42-,43-,45+,46-,53-,54+/m0/s1 |
|---|
| InChI Key | WUALHADVSUQRQJ-IVKAJXLBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydrolyzable tannins. These are tannins with a structure characterized by either of the following models. In model 1, the structure contains galloyl units (in some cases, shikimic acid units) that are linked to diverse polyol carbohydrate-, catechin-, or triterpenoid units. In model 2, contains at least two galloyl units C-C coupled to each other, and do not contain a glycosidically linked catechin unit. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Tannins |
|---|
| Sub Class | Hydrolyzable tannins |
|---|
| Direct Parent | Hydrolyzable tannins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydrolyzable tannin
- Hexacarboxylic acid or derivatives
- Galloyl ester
- Gallic acid or derivatives
- P-hydroxybenzoic acid alkyl ester
- M-hydroxybenzoic acid ester
- P-hydroxybenzoic acid ester
- Dihydroxybenzoic acid
- Diaryl ether
- Benzoate ester
- Pyrogallol derivative
- Benzoic acid or derivatives
- Benzenetriol
- Phenoxy compound
- Phenol ether
- Catechol
- Benzoyl
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Benzenoid
- Oxane
- Monosaccharide
- Monocyclic benzene moiety
- Secondary alcohol
- Lactone
- Carboxylic acid ester
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Ether
- Carboxylic acid derivative
- Acetal
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uk9-1921201211-fdb0c413b23a3984c5bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uk9-0910100100-88e2065a88fdbfdebdcc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kmi-3925300000-01e49e8ff441d8f9adb7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0gb9-1950100220-1144915f67103242edd7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gb9-3930202110-257b7f44940605ab2d64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-1924213000-2037c8ab33ca89ea68ce | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|