| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:29:54 UTC |
|---|
| Update Date | 2016-11-09 01:20:44 UTC |
|---|
| Accession Number | CHEM032557 |
|---|
| Identification |
|---|
| Common Name | Pyro-L-glutaminyl-L-glutamine |
|---|
| Class | Small Molecule |
|---|
| Description | A dpeptide obtained by formal condensation of the carboxy group of L-pyroglutamine with the amino group of L-glutamine |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
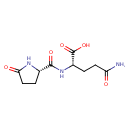
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-Oxoprolylglutamine | ChEBI | | L-p-Glu-L-GLN | ChEBI | | L-Pyroglutamyl-L-glutamine | ChEBI | | p-Glu-GLN | ChEBI | | Pyroglutaminylglutamine | Dictionary of Food Compounds, HMDB | | N2-(5-Oxoprolyl)glutamine | Dictionary of Food Compounds, HMDB | | N2-L-Pyroglutamyl-L-glutamine | HMDB | | N2-(5-Oxo-L-prolyl)-L-glutamine | HMDB | | 5-Oxo-L-prolyl-L-glutamine | HMDB | | Pyro-L-glutaminyl-L-glutamine | HMDB, ChEBI |
|
|---|
| Chemical Formula | C10H15N3O5 |
|---|
| Average Molecular Mass | 257.243 g/mol |
|---|
| Monoisotopic Mass | 257.101 g/mol |
|---|
| CAS Registry Number | 109481-23-4 |
|---|
| IUPAC Name | (2S)-4-carbamoyl-2-{[(2S)-5-oxopyrrolidin-2-yl]formamido}butanoic acid |
|---|
| Traditional Name | (2S)-4-carbamoyl-2-{[(2S)-5-oxopyrrolidin-2-yl]formamido}butanoic acid |
|---|
| SMILES | NC(=O)CC[C@H](NC(=O)[C@@H]1CCC(=O)N1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H15N3O5/c11-7(14)3-1-6(10(17)18)13-9(16)5-2-4-8(15)12-5/h5-6H,1-4H2,(H2,11,14)(H,12,15)(H,13,16)(H,17,18)/t5-,6-/m0/s1 |
|---|
| InChI Key | ILAITOFTZJRIFJ-WDSKDSINSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as o-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a O-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | O-glycosyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Disaccharide
- O-glycosyl compound
- Tyrosol derivative
- Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Oxane
- Benzenoid
- Secondary alcohol
- Organoheterocyclic compound
- Oxacycle
- Acetal
- Polyol
- Alcohol
- Hydrocarbon derivative
- Primary alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-06ry-2490000000-c28b669dbdcd3b5724db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001j-8930000000-83e047e639e90bbeba8e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f8a-9200000000-2c96198f0aba41624530 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-1290000000-02c28f877a8856526b0a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-06to-5950000000-2c384a082797953a3fbf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-3b457826471f4562c2ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0btc-1390000000-af0c102cac44bca29f76 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gx0-5930000000-20b560ddb59d3326f42a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9300000000-5d94a693694dc4964f06 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a6r-0690000000-dd1451bb3adcb7fe3a59 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-4900000000-0bdeee3106c87c458470 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-76686f931ba989ac888c | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039229 |
|---|
| FooDB ID | FDB018762 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9185807 |
|---|
| ChEBI ID | 88956 |
|---|
| PubChem Compound ID | 11010621 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|