| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:29:41 UTC |
|---|
| Update Date | 2016-11-09 01:20:44 UTC |
|---|
| Accession Number | CHEM032551 |
|---|
| Identification |
|---|
| Common Name | N-(gamma-Glutamyl)ethanolamine |
|---|
| Class | Small Molecule |
|---|
| Description | N-(gamma-Glutamyl)ethanolamine is found in mushrooms. N-(gamma-Glutamyl)ethanolamine is a constituent of the fruiting body of Agaricus bisporus (button mushroom). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
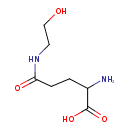
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N-(g-Glutamyl)ethanolamine | Generator | | N-(Γ-glutamyl)ethanolamine | Generator | | N-(g-Glutamyl)ethanolamine, 8ci | HMDB | | 2-Amino-4-[(2-hydroxyethyl)-C-hydroxycarbonimidoyl]butanoate | HMDB |
|
|---|
| Chemical Formula | C7H14N2O4 |
|---|
| Average Molecular Mass | 190.197 g/mol |
|---|
| Monoisotopic Mass | 190.095 g/mol |
|---|
| CAS Registry Number | 2650-74-0 |
|---|
| IUPAC Name | 2-amino-4-[(2-hydroxyethyl)carbamoyl]butanoic acid |
|---|
| Traditional Name | 2-amino-4-[(2-hydroxyethyl)carbamoyl]butanoic acid |
|---|
| SMILES | [H][C@](N)(CCC(=O)NCCO)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C7H14N2O4/c8-5(7(12)13)1-2-6(11)9-3-4-10/h5,10H,1-4,8H2,(H,9,11)(H,12,13)/t5-/m0/s1 |
|---|
| InChI Key | DGBJQQBLTDLFMF-YFKPBYRVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glutamine and derivatives. Glutamine and derivatives are compounds containing glutamine or a derivative thereof resulting from reaction of glutamine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Glutamine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glutamine or derivatives
- Alpha-amino acid
- N-acylethanolamine
- Fatty amide
- N-acyl-amine
- Fatty acid
- Fatty acyl
- Carboxamide group
- Secondary carboxylic acid amide
- Amino acid
- Alkanolamine
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Hydrocarbon derivative
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Primary aliphatic amine
- Organic oxygen compound
- Primary alcohol
- Alcohol
- Carbonyl group
- Amine
- Organic nitrogen compound
- Primary amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01pp-9500000000-a1afaca1b713b9a47d6d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0fka-9860000000-46d1f7e57c68641fb47f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01vn-2900000000-5f8ebe72d741f0623732 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dj-9500000000-934f629ea7005dba454e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9100000000-2f54b5a7e66cb334b907 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-6052896ab4953225c91b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-022i-4900000000-cea2cc53e3a1ac927175 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-8139443913d5c4b9d108 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00bi-0900000000-8c480f454247ec6846c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004v-5900000000-20ab62dd8fe2d9d51397 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-bb704b8b1bcf627ae1fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01vo-4900000000-f914717658973a849545 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9300000000-d95f3e3eec1236f3460b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06rx-9100000000-f65cf25f26f103fae12a | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039222 |
|---|
| FooDB ID | FDB018754 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8373507 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10198007 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|