| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:29:28 UTC |
|---|
| Update Date | 2016-11-09 01:20:44 UTC |
|---|
| Accession Number | CHEM032548 |
|---|
| Identification |
|---|
| Common Name | 1,3,5,8-Tetrahydroxy-6-methoxy-2-methylanthraquinone 8-O-b-D-glucoside |
|---|
| Class | Small Molecule |
|---|
| Description | 1,3,5,8-Tetrahydroxy-6-methoxy-2-methylanthraquinone 8-O-b-D-glucoside is found in fruits. 1,3,5,8-Tetrahydroxy-6-methoxy-2-methylanthraquinone 8-O-b-D-glucoside is isolated from Diospyros discolor (mabolo). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
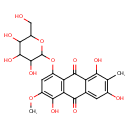
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Methoxy-4-phenylpyridinium iodide | HMDB | | Pyridinium, 1-methoxy-4-phenyl-, iodide | HMDB | | Pyridinium, 1-methoxy-4-phenyl-, iodide (1:1) | HMDB |
|
|---|
| Chemical Formula | C22H22O12 |
|---|
| Average Molecular Mass | 478.403 g/mol |
|---|
| Monoisotopic Mass | 478.111 g/mol |
|---|
| CAS Registry Number | 101508-15-0 |
|---|
| IUPAC Name | 1,3,5-trihydroxy-6-methoxy-2-methyl-8-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-9,10-dihydroanthracene-9,10-dione |
|---|
| Traditional Name | 1,3,5-trihydroxy-6-methoxy-2-methyl-8-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}anthracene-9,10-dione |
|---|
| SMILES | COC1=C(O)C2=C(C(=O)C3=C(O)C(C)=C(O)C=C3C2=O)C(OC2OC(CO)C(O)C(O)C2O)=C1 |
|---|
| InChI Identifier | InChI=1S/C22H22O12/c1-6-8(24)3-7-12(15(6)25)19(29)13-9(4-10(32-2)17(27)14(13)16(7)26)33-22-21(31)20(30)18(28)11(5-23)34-22/h3-4,11,18,20-25,27-28,30-31H,5H2,1-2H3 |
|---|
| InChI Key | VAYMARBXYQUXAO-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxyanthraquinones. Hydroxyanthraquinones are compounds containing a hydroxyanthraquinone moiety, which consists of an anthracene bearing a quinone, and hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Anthracenes |
|---|
| Sub Class | Anthraquinones |
|---|
| Direct Parent | Hydroxyanthraquinones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxyanthraquinone
- Phenolic glycoside
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Anisole
- Aryl ketone
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Monosaccharide
- Oxane
- Vinylogous acid
- Ketone
- Secondary alcohol
- Polyol
- Acetal
- Organoheterocyclic compound
- Oxacycle
- Ether
- Alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Primary alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-08mi-9303700000-13319050dfee7d6568da | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-057i-2331029000-6ebb644cbe9e2224ee73 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-02vj-0137900000-76aa4e9232f5a90e17e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014j-0879100000-e2521ea3a1972ef1428e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fr2-3596000000-a47b529faa05134be609 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00or-1215900000-df614bb7132b46372811 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-1269300000-0a63ad0013a89b321ed2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ke-3194000000-9e63799458fe8482deee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000900000-fb9c3806532ee9ca56be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00or-1037900000-2377ba8f6ba479824802 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05r1-5094400000-da5df5f937970590878d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00or-0007900000-4f1552621a2480a0cd39 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0019300000-bd56feca7d3a7bfaab76 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-016u-4119100000-fb66bc4b95b9bfc6d3cf | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039211 |
|---|
| FooDB ID | FDB018742 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752578 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|