| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:27:01 UTC |
|---|
| Update Date | 2016-11-09 01:20:44 UTC |
|---|
| Accession Number | CHEM032514 |
|---|
| Identification |
|---|
| Common Name | 1,6-Digalloyl-beta-D-glucopyranose |
|---|
| Class | Small Molecule |
|---|
| Description | 1,6-Digalloyl-beta-D-glucopyranose is found in green vegetables. 1,6-Digalloyl-beta-D-glucopyranose is present in commercial rhubarb. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
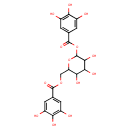
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,6-Digalloyl-b-D-glucopyranose | Generator | | 1,6-Digalloyl-β-D-glucopyranose | Generator | | 4,4'-Stilbenedicarboxamidine, dihydrochloride | HMDB | | 4-Quinoxaline mono-N-oxide | HMDB | | Quinoxaline 1-oxide | HMDB | | Quinoxaline mono-N-oxide | HMDB | | Quinoxaline monooxide | HMDB | | Quinoxaline N-oxide | HMDB | | Quinoxaline, 1-oxide | HMDB | | Quinoxaline, N-monooxide | HMDB | | [3,4,5-Trihydroxy-6-(3,4,5-trihydroxybenzoyloxy)oxan-2-yl]methyl 3,4,5-trihydroxybenzoic acid | Generator |
|
|---|
| Chemical Formula | C20H20O14 |
|---|
| Average Molecular Mass | 484.364 g/mol |
|---|
| Monoisotopic Mass | 484.085 g/mol |
|---|
| CAS Registry Number | 23363-08-8 |
|---|
| IUPAC Name | [3,4,5-trihydroxy-6-(3,4,5-trihydroxybenzoyloxy)oxan-2-yl]methyl 3,4,5-trihydroxybenzoate |
|---|
| Traditional Name | [3,4,5-trihydroxy-6-(3,4,5-trihydroxybenzoyloxy)oxan-2-yl]methyl 3,4,5-trihydroxybenzoate |
|---|
| SMILES | OC1C(O)C(COC(=O)C2=CC(O)=C(O)C(O)=C2)OC(OC(=O)C2=CC(O)=C(O)C(O)=C2)C1O |
|---|
| InChI Identifier | InChI=1S/C20H20O14/c21-8-1-6(2-9(22)13(8)25)18(30)32-5-12-15(27)16(28)17(29)20(33-12)34-19(31)7-3-10(23)14(26)11(24)4-7/h1-4,12,15-17,20-29H,5H2 |
|---|
| InChI Key | LYGRISUQIZNHGM-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tannins. These are naturally occurring polyphenols which be categorized into four main classes: hydrolyzable tannin (based on ellagic acid or gallic acid), condensed tannins (made of oligomeric or polymeric proanthocyanidins), complex tannins (made of a catechin bound to a gallotannin or elagitannin), and phlorotannins (oligomers of phloroglucinol). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Tannins |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Tannins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tannin
- Galloyl ester
- Gallic acid or derivatives
- P-hydroxybenzoic acid alkyl ester
- M-hydroxybenzoic acid ester
- P-hydroxybenzoic acid ester
- Benzoate ester
- Benzenetriol
- Benzoic acid or derivatives
- Pyrogallol derivative
- Benzoyl
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Monosaccharide
- Benzenoid
- Oxane
- Dicarboxylic acid or derivatives
- Carboxylic acid ester
- Secondary alcohol
- Acetal
- Oxacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Polyol
- Hydrocarbon derivative
- Alcohol
- Organic oxygen compound
- Organic oxide
- Organooxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-2955300000-87a757c40caeaf5c2f6d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0w29-2931804000-260e5add687fea243ba3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , negative | splash10-0089-0472900000-cdb7559c7a99bbf752cc | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-0udi-0910000000-664a882244a97c7d24fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gbi-0917800000-c457dceb1da2a4c0f60d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uxr-0912100000-8947c60a980f8633b051 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-1900000000-cdf97965c27b0edf9ae7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0159-0912500000-2bd15c1295338c2b9310 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0901000000-db11dd1164818be5d87e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-1900000000-807e365640c3b6686539 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0102900000-cc5fe0b3baded708e25f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0i00-0944400000-6e07fdc9b2392336747c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-1901000000-19320c222ed82aeda567 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fri-0826900000-a1e167060e6a0519fddc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uy0-0933400000-1301f6634e43a4b6b3e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-0910200000-8e161b498d000528c0c8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039179 |
|---|
| FooDB ID | FDB018707 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00037193 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 2579268 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 3332212 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|