| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:26:11 UTC |
|---|
| Update Date | 2016-11-09 01:20:44 UTC |
|---|
| Accession Number | CHEM032495 |
|---|
| Identification |
|---|
| Common Name | L-D-alpha-Glutamylalanine |
|---|
| Class | Small Molecule |
|---|
| Description | A dipeptide formed from L-alpha-glutamyl and L-alanine residues. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
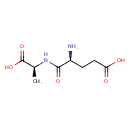
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| alpha-Glu-ala | ChEBI | | alpha-Glutamylalanine | ChEBI | | EA | ChEBI | | L-alpha-Glu-L-ala | ChEBI | | L-Glu-L-ala | ChEBI | | N-L-alpha-Glutamyl-L-alanine | ChEBI | | a-Glu-ala | Generator | | Α-glu-ala | Generator | | a-Glutamylalanine | Generator | | Α-glutamylalanine | Generator | | L-a-Glu-L-ala | Generator | | L-Α-glu-L-ala | Generator | | N-L-a-Glutamyl-L-alanine | Generator | | N-L-Α-glutamyl-L-alanine | Generator | | a-L-Glutamyl-L-alanine | HMDB | | alpha-L-Glutamyl-L-alanine | HMDB | | Glu-ala | HMDB | | L-alpha-Glutamyl-L-amino acid | HMDB | | L-Glutamyl-L-alanine | HMDB | | Α-L-glu-L-ala | HMDB | | Α-L-glutamyl-L-alanine | HMDB | | L-Α-glutamyl-L-alanine | HMDB | | N-Α-glutamylalanine | HMDB | | N-Α-L-glutamyl-L-alanine | HMDB | | N-L-Α-glutamylalanine | HMDB | | alpha-L-Glu-L-ala | HMDB | | L-alpha-Glutamyl-L-alanine | HMDB | | N-alpha-Glutamylalanine | HMDB | | N-alpha-L-Glutamyl-L-alanine | HMDB | | N-L-alpha-Glutamylalanine | HMDB | | 4-Amino-N-(1-carboxyethyl)-glutaramic acid | HMDB | | NSC 334200 | HMDB | | N-Glutamylalanine | HMDB | | N-L-Glutamyl-L-alanine | HMDB | | Glutamyl-alanine | HMDB | | Glutamic acid alanine dipeptide | HMDB | | Glutamate alanine dipeptide | HMDB | | Glutamic acid-alanine dipeptide | HMDB | | Glutamate-alanine dipeptide | HMDB | | e-a Dipeptide | HMDB | | EA dipeptide | HMDB | | Glutamylalanine | HMDB, ChEBI |
|
|---|
| Chemical Formula | C8H14N2O5 |
|---|
| Average Molecular Mass | 218.207 g/mol |
|---|
| Monoisotopic Mass | 218.090 g/mol |
|---|
| CAS Registry Number | 76186-47-5 |
|---|
| IUPAC Name | (4S)-4-amino-4-{[(1S)-1-carboxyethyl]carbamoyl}butanoic acid |
|---|
| Traditional Name | glutamylalanine |
|---|
| SMILES | CC(NC(=O)C(N)CCC(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C8H14N2O5/c1-4(8(14)15)10-7(13)5(9)2-3-6(11)12/h4-5H,2-3,9H2,1H3,(H,10,13)(H,11,12)(H,14,15) |
|---|
| InChI Key | JZDHUJAFXGNDSB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- Glutamic acid or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- N-acyl-l-alpha-amino acid
- Alpha-amino acid amide
- Alanine or derivatives
- Alpha-amino acid or derivatives
- Amino fatty acid
- N-acyl-amine
- Fatty acyl
- Fatty acid
- Dicarboxylic acid or derivatives
- Fatty amide
- Amino acid
- Carboxamide group
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Carboxylic acid
- Organic nitrogen compound
- Primary aliphatic amine
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Amine
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ue9-2890000000-c4ee77e01b7d1e1d3383 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f6x-9710000000-fed83af06726ad8e0fc9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-f57412a046d4f71bc28e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-1790000000-2d05f958e7b0df336b72 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9800000000-5337ff8f303b11c163a1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000f-9100000000-bec827e77d19d9072030 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-9310000000-5568c656cbd88eec1e21 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f8i-9200000000-179a8f0ba0ceefc9c920 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9100000000-578dc95b4dcb61269358 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0003764 |
|---|
| FooDB ID | FDB018687 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 5360636 |
|---|
| ChEBI ID | 73849 |
|---|
| PubChem Compound ID | 6992506 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Sachs, Howard; Brand, Erwin. Optical rotation of peptides. VIII. Glutamic acid tripeptides. Journal of the American Chemical Society (1954), 76 1811-14. | | 2. King GF, Kuchel PW: Assimilation of alpha-glutamyl-peptides by human erythrocytes. A possible means of glutamate supply for glutathione synthesis. Biochem J. 1985 May 1;227(3):833-42. | | 3. Friedrichsen GM, Jakobsen P, Taub M, Begtrup M: Application of enzymatically stable dipeptides for enhancement of intestinal permeability. Synthesis and in vitro evaluation of dipeptide-coupled compounds. Bioorg Med Chem. 2001 Oct;9(10):2625-32. | | 4. Kanazawa A, Kakimoto Y, Nakajima T, Sano I: Identification of gamma-glutamylserine, gamma-glutamylalanine, gamma-glutamylvaline and S-methylglutathione of bovine brain. Biochim Biophys Acta. 1965 Nov 15;111(1):90-5. | | 5. Conway de Macario E, Macario AJ, Magarinos MC, Konig H, Kandler O: Dissecting the antigenic mosaic of the Archaebacterium Methanobacterium thermoautotrophicum by monoclonal antibodies of defined molecular specificity. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6346-50. | | 6. Kondo H, Hashimoto M, Nagata K, Tomita K, Tsubota H: Assay of gamma-glutamyltransferase with amino acid dehydrogenases from Bacillus stearothermophilus as auxiliary enzymes. Clin Chim Acta. 1992 Apr 30;207(1-2):1-9. | | 7. Konig H, Kandler O, Jensen M, Rietschel ET: The primary structure of the glycan moiety of pseudomurein from Methanobacterium thermoautotrophicum. Hoppe Seylers Z Physiol Chem. 1983 Jun;364(6):627-36. | | 8. Fukuda M, Ogawa T, Sasaoka K: Optical configuration of -glutamylalanine in pea seedlings. Biochim Biophys Acta. 1973 Apr 28;304(2):363-6. |
|
|---|