| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:25:11 UTC |
|---|
| Update Date | 2016-11-09 01:20:43 UTC |
|---|
| Accession Number | CHEM032473 |
|---|
| Identification |
|---|
| Common Name | Hericenone E |
|---|
| Class | Small Molecule |
|---|
| Description | Hericenone E is found in mushrooms. Hericenone E is from the edible lion's mane mushroom (Hericium erinaceum). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
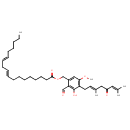
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-(3',7'-Dimethyl-5'-oxo-2',6'-octadienyl)-2-formyl-3-hydroxy-5-methoxybenzyl-linoleate | HMDB | | [4-(3,7-Dimethyl-5-oxoocta-2,6-dien-1-yl)-2-formyl-3-hydroxy-5-methoxyphenyl]methyl (9E,12E)-octadeca-9,12-dienoic acid | Generator |
|
|---|
| Chemical Formula | C37H54O6 |
|---|
| Average Molecular Mass | 594.821 g/mol |
|---|
| Monoisotopic Mass | 594.392 g/mol |
|---|
| CAS Registry Number | 137592-05-3 |
|---|
| IUPAC Name | {4-[(2E)-3,7-dimethyl-5-oxoocta-2,6-dien-1-yl]-2-formyl-3-hydroxy-5-methoxyphenyl}methyl (9E,12E)-octadeca-9,12-dienoate |
|---|
| Traditional Name | {4-[(2E)-3,7-dimethyl-5-oxoocta-2,6-dien-1-yl]-2-formyl-3-hydroxy-5-methoxyphenyl}methyl (9E,12E)-octadeca-9,12-dienoate |
|---|
| SMILES | CCCCC\C=C\C\C=C\CCCCCCCC(=O)OCC1=CC(OC)=C(C\C=C(/C)CC(=O)C=C(C)C)C(O)=C1C=O |
|---|
| InChI Identifier | InChI=1S/C37H54O6/c1-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-36(40)43-28-31-26-35(42-5)33(37(41)34(31)27-38)23-22-30(4)25-32(39)24-29(2)3/h10-11,13-14,22,24,26-27,41H,6-9,12,15-21,23,25,28H2,1-5H3/b11-10+,14-13+,30-22+ |
|---|
| InChI Key | SUAXEWQRYKSWIW-JWUQTBJASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as lineolic acids and derivatives. These are derivatives of lineolic acid. Lineolic acid is a polyunsaturated omega-6 18 carbon long fatty acid, with two CC double bonds at the 9- and 12-positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Lineolic acids and derivatives |
|---|
| Direct Parent | Lineolic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Octadecanoid
- Aromatic monoterpenoid
- Benzyloxycarbonyl
- Methoxyphenol
- Monocyclic monoterpenoid
- Monoterpenoid
- Hydroxybenzaldehyde
- Methoxybenzene
- Benzaldehyde
- Phenol ether
- Benzoyl
- Anisole
- Phenoxy compound
- 1-hydroxy-4-unsubstituted benzenoid
- Aryl-aldehyde
- Phenol
- Fatty acid ester
- Alkyl aryl ether
- Benzenoid
- Monocyclic benzene moiety
- Enone
- Vinylogous acid
- Acryloyl-group
- Alpha,beta-unsaturated ketone
- Carboxylic acid ester
- Ketone
- Ether
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Carbonyl group
- Aldehyde
- Organooxygen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-06si-9453230000-59c58e53abbcd7e4a5a7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0uei-9352013000-cac92bc269f7091e9460 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Hericenone E,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-02bb-0043190000-9b2c4dfd94f97aa11761 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02t9-2192220000-d937271505d107861431 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00lr-3190010000-87c6d0f89324e66b8e99 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01ox-1071090000-706015d4dea36c791ced | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fr-3092030000-307c93c6db5ba237a770 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0btc-8094020000-f7da776ba4a8e69e03bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014j-1165090000-cd558421bbab839945ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-017i-3593000000-7bb4ad960c5f68cf571e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-3296000000-5300dd9531eaac6c55e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0068090000-8d29ef22adb102cf5be1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-016r-0091010000-1c3d500a4ca47f9499b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fy9-0091160000-8ddf4bb20f14742dca0b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039140 |
|---|
| FooDB ID | FDB018660 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00023977 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30777326 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752559 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|