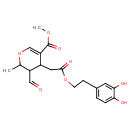| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:25:03 UTC |
|---|
| Update Date | 2016-11-09 01:20:43 UTC |
|---|
| Accession Number | CHEM032470 |
|---|
| Identification |
|---|
| Common Name | (3,4-Dihydroxyphenylethyl)-elenaiate |
|---|
| Class | Small Molecule |
|---|
| Description | 2-(3,4-Dihydroxyphenylethyl)-6-epi-elenaiate is found in fruits. 2-(3,4-Dihydroxyphenylethyl)-6-epi-elenaiate is isolated from olive leaves (Olea europaea). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(3,4-Dihydroxyphenylethyl)-6-epi-elenaiic acid | Generator | | Methyl 4-{2-[2-(3,4-dihydroxyphenyl)ethoxy]-2-oxoethyl}-3-formyl-2-methyl-3,4-dihydro-2H-pyran-5-carboxylic acid | HMDB | | (3,4-Dihydroxyphenylethyl)-elenaiic acid | Generator |
|
|---|
| Chemical Formula | C19H22O8 |
|---|
| Average Molecular Mass | 378.373 g/mol |
|---|
| Monoisotopic Mass | 378.131 g/mol |
|---|
| CAS Registry Number | 50915-70-3 |
|---|
| IUPAC Name | methyl 4-{2-[2-(3,4-dihydroxyphenyl)ethoxy]-2-oxoethyl}-3-formyl-2-methyl-3,4-dihydro-2H-pyran-5-carboxylate |
|---|
| Traditional Name | methyl 4-{2-[2-(3,4-dihydroxyphenyl)ethoxy]-2-oxoethyl}-5-formyl-6-methyl-5,6-dihydro-4H-pyran-3-carboxylate |
|---|
| SMILES | COC(=O)C1=COC(C)C(C=O)C1CC(=O)OCCC1=CC=C(O)C(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C19H22O8/c1-11-14(9-20)13(15(10-27-11)19(24)25-2)8-18(23)26-6-5-12-3-4-16(21)17(22)7-12/h3-4,7,9-11,13-14,21-22H,5-6,8H2,1-2H3 |
|---|
| InChI Key | DEBZOPZQKONWTK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tyrosols and derivatives. Tyrosols and derivatives are compounds containing a hydroxyethyl group attached to the C4 carbon of a phenol group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Tyrosols and derivatives |
|---|
| Direct Parent | Tyrosols and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tyrosol derivative
- Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Dicarboxylic acid or derivatives
- Methyl ester
- Vinylogous ester
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Carboxylic acid ester
- Oxacycle
- Carboxylic acid derivative
- Organoheterocyclic compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Aldehyde
- Carbonyl group
- Organic oxygen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0002-1923000000-b162c57c609ff6129db1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0a4i-2590470000-d0dba7e92650fd226735 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0659000000-e34b5c78f5ccd131d534 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0941000000-d5557b527cccab58ba73 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-6911000000-ffa78208e9a185cabf67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00b9-1569000000-def5f49d19cc02c55a86 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006x-0894000000-048dc0e2c9ec4e183dfd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abi-0900000000-88157ae69691f260fc86 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03fr-0019000000-e2ee6ddebacb70bf7440 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014r-0719000000-10781e8bb459a83c96b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-016r-5902000000-ae2d749ce9574e28101e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0029000000-e0a6b501d09d6d906740 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00rl-0953000000-c58e426532e20f76e919 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dr-1910000000-d1d649fa68cf1b022dbb | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039137 |
|---|
| FooDB ID | FDB018656 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 18953410 |
|---|
| ChEBI ID | 175849 |
|---|
| PubChem Compound ID | 18684079 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|