| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:24:39 UTC |
|---|
| Update Date | 2016-11-09 01:20:43 UTC |
|---|
| Accession Number | CHEM032461 |
|---|
| Identification |
|---|
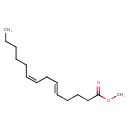
| Common Name | Methyl (Z,Z)-5,8-tetradecadienoate |
|---|
| Class | Small Molecule |
|---|
| Description | Methyl (Z,Z)-5,8-tetradecadienoate is found in pomes. Methyl (Z,Z)-5,8-tetradecadienoate is a constituent of pear volatiles. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Methyl (Z,Z)-5,8-tetradecadienoic acid | Generator | | Methyl (5E,8Z)-tetradeca-5,8-dienoic acid | Generator |
|
|---|
| Chemical Formula | C15H26O2 |
|---|
| Average Molecular Mass | 238.366 g/mol |
|---|
| Monoisotopic Mass | 238.193 g/mol |
|---|
| CAS Registry Number | 18829-89-5 |
|---|
| IUPAC Name | methyl (5E,8Z)-tetradeca-5,8-dienoate |
|---|
| Traditional Name | methyl (5E,8Z)-tetradeca-5,8-dienoate |
|---|
| SMILES | CCCCC\C=C/C\C=C\CCCC(=O)OC |
|---|
| InChI Identifier | InChI=1S/C15H26O2/c1-3-4-5-6-7-8-9-10-11-12-13-14-15(16)17-2/h7-8,10-11H,3-6,9,12-14H2,1-2H3/b8-7-,11-10+ |
|---|
| InChI Key | UZFMPMFXZHNDLT-QEFCTBRHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fatty acid methyl esters. Fatty acid methyl esters are compounds containing a fatty acid that is esterified with a methyl group. They have the general structure RC(=O)OR', where R=fatty aliphatic tail or organyl group and R'=methyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty acid esters |
|---|
| Direct Parent | Fatty acid methyl esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Fatty acid methyl ester
- Methyl ester
- Carboxylic acid ester
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0abd-6910000000-6b14fdcf23f76ef139b0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0290000000-16a234eb29eae3ec965a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0639-6940000000-c5165d7814343ea47dfb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9400000000-a369cb30f9a3f8a5d568 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-cca4c569f6c864b3695e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052r-1090000000-f4ecb6698060a61c956b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9320000000-fbfe8c605dfd28252904 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4r-0090000000-241a6b765ff4e910728f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052r-1190000000-cf2a0b1a61d5ecae8baa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0abc-9310000000-94caff06853f2778264b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0569-9630000000-17651c036656a417a21a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05o1-9100000000-1d3073601dfeb4a8dc21 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-067l-9000000000-4b2d4c1f73aaf6b6f8a2 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039130 |
|---|
| FooDB ID | FDB018643 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30777324 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 12813433 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|