| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:24:28 UTC |
|---|
| Update Date | 2016-11-09 01:20:43 UTC |
|---|
| Accession Number | CHEM032459 |
|---|
| Identification |
|---|
| Common Name | (3'RS,3'SR)-Astaxanthin |
|---|
| Class | Small Molecule |
|---|
| Description | A carotenone that consists of beta,beta-carotene-4,4'-dione bearing two hydroxy substituents at positions 3 and 3' (the 3S,3'S diastereomer). A carotenoid pigment found mainly in animals (crustaceans, echinoderms) but also occurring in plants. It can occur free (as a red pigment), as an ester, or as a blue, brown or green chromoprotein. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
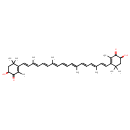
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (3S,3's)-Astaxanthin | ChEBI | | 3,3'-Dihydroxy-beta,beta-carotene-4,4'-dione | ChEBI | | 3,3'-Dihydroxy-beta-carotene-4,4'-dione | ChEBI | | all-trans-(3S,3's)-Astaxanthin | ChEBI | | Astaxanthine | ChEBI | | e 161J | ChEBI | | Ovoester | ChEBI | | (3S,3's)-3,3'-Dihydroxy-beta,beta-carotene-4,4'-dione | Kegg | | 3,3'-Dihydroxy-b,b-carotene-4,4'-dione | Generator | | 3,3'-Dihydroxy-β,β-carotene-4,4'-dione | Generator | | 3,3'-Dihydroxy-b-carotene-4,4'-dione | Generator | | 3,3'-Dihydroxy-β-carotene-4,4'-dione | Generator | | (3S,3's)-3,3'-Dihydroxy-b,b-carotene-4,4'-dione | Generator | | (3S,3's)-3,3'-Dihydroxy-β,β-carotene-4,4'-dione | Generator | | (3S,3's)-all-trans-Astaxanthin | HMDB | | all-trans-3,3'-Dihydroxy-b-carotene-4,4'-dione (8ci) | HMDB | | all-trans-3,3'-Dihydroxy-beta-carotene-4,4'-dione (8ci) | HMDB | | all-trans-Astaxanthin | HMDB | | AstaREAL | HMDB | | Astaxanthin (6ci) | HMDB | | BioAstin | HMDB | | BioAstin oleoresin | HMDB | | Carophyll pink | HMDB | | Lucantin pink | HMDB | | Natupink | HMDB | | trans-Astaxanthin | HMDB | | e-Astaxanthin | HMDB | | (3S,3’S)-3,3’-dihydroxy-β,β-carotene-4,4’-dione | HMDB | | (3S,3’S)-astaxanthin | HMDB | | (3S,3’S)-all-trans-astaxanthin | HMDB | | (S,S)-Astaxanthin | HMDB | | all-trans-(3S,3’S)-astaxanthin | HMDB | | Astaxanthin | HMDB |
|
|---|
| Chemical Formula | C40H52O4 |
|---|
| Average Molecular Mass | 596.839 g/mol |
|---|
| Monoisotopic Mass | 596.387 g/mol |
|---|
| CAS Registry Number | 71772-51-5 |
|---|
| IUPAC Name | (6S)-6-hydroxy-3-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(4S)-4-hydroxy-2,6,6-trimethyl-3-oxocyclohex-1-en-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaen-1-yl]-2,4,4-trimethylcyclohex-2-en-1-one |
|---|
| Traditional Name | astaxanthin |
|---|
| SMILES | C\C(\C=C\C=C(/C)\C=C\C1=C(C)C(=O)C(O)CC1(C)C)=C\C=C\C=C(/C)\C=C\C=C(/C)\C=C\C1=C(C)C(=O)C(O)CC1(C)C |
|---|
| InChI Identifier | InChI=1S/C40H52O4/c1-27(17-13-19-29(3)21-23-33-31(5)37(43)35(41)25-39(33,7)8)15-11-12-16-28(2)18-14-20-30(4)22-24-34-32(6)38(44)36(42)26-40(34,9)10/h11-24,35-36,41-42H,25-26H2,1-10H3/b12-11+,17-13+,18-14+,23-21+,24-22+,27-15-,28-16+,29-19+,30-20+ |
|---|
| InChI Key | MQZIGYBFDRPAKN-HDQLMXHPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthophylls. These are carotenoids containing an oxygenated carotene backbone. Carotenes are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. Xanthophylls arise by oxygenation of the carotene backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Tetraterpenoids |
|---|
| Direct Parent | Xanthophylls |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthophyll
- Cyclohexenone
- Cyclic ketone
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-2000090000-c6336112eb898c2f0a48 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0udi-3000019000-6b5720849187b199d6bf | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Astaxanthin,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0002-0961030000-dc7a74c186ee85f204fa | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0002-0961030000-368bb77cba4aef27a5cb | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0000090000-ce16b33770cb7a4b2ff7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0232390000-f37e3530214c99f63306 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-1296830000-ac8ea41a53a053acb7c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03fr-0185910000-7af6a00762ad7e8ee75d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0000090000-248171e80348a7e1d17e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0000090000-b9759de23c7494bb7b2d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-0333390000-074ea5de5d017bafdb5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004m-0142590000-4e72a5bda186fd51912c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03fr-0195650000-3ee8ba797d4a2fb9c80f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01u3-0013900000-71e9a6336700d9e8fca6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0001090000-836dc358b0a566457c26 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004j-0117190000-07d5d205542df36e4f5b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002b-0239230000-7be6270484a4c641a468 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB06543 |
|---|
| HMDB ID | HMDB0002204 |
|---|
| FooDB ID | FDB018640 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00000918 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 3671 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Astaxanthin |
|---|
| Chemspider ID | 4444636 |
|---|
| ChEBI ID | 40968 |
|---|
| PubChem Compound ID | 5281224 |
|---|
| Kegg Compound ID | C08580 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | M2MDB005295 |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. https://www.ncbi.nlm.nih.gov/pubmed/?term=21833799 | | 2. https://www.ncbi.nlm.nih.gov/pubmed/?term=21883294 | | 3. https://www.ncbi.nlm.nih.gov/pubmed/?term=22119431 | | 4. https://www.ncbi.nlm.nih.gov/pubmed/?term=22188802 | | 5. https://www.ncbi.nlm.nih.gov/pubmed/?term=22189778 | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=22221991 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=22267192 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=22279065 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=22309505 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=22349894 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=22406426 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=22428137 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=22432539 | | 14. https://www.ncbi.nlm.nih.gov/pubmed/?term=22455145 | | 15. Berg, Michael; Essl, Stefan; Hugentobler, Max. Process for the preparation of astaxanthin. PCT Int. Appl. (2007), 19pp. | | 16. Berg, Michael; Essl, Stefan; Hugentobler, Max. Process for the preparation of astaxanthin. PCT Int. Appl. (2007), 19pp. | | 17. Hussein G, Sankawa U, Goto H, Matsumoto K, Watanabe H: Astaxanthin, a carotenoid with potential in human health and nutrition. J Nat Prod. 2006 Mar;69(3):443-9. | | 18. Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM: Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr. 2006;46(2):185-96. | | 19. Simons K, Toomre D: Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000 Oct;1(1):31-9. | | 20. Watson AD: Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006 Oct;47(10):2101-11. Epub 2006 Aug 10. | | 21. Sethi JK, Vidal-Puig AJ: Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007 Jun;48(6):1253-62. Epub 2007 Mar 20. | | 22. Lingwood D, Simons K: Lipid rafts as a membrane-organizing principle. Science. 2010 Jan 1;327(5961):46-50. doi: 10.1126/science.1174621. | | 23. Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC. | | 24. The lipid handbook with CD-ROM |
|
|---|