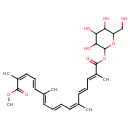| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:24:07 UTC |
|---|
| Update Date | 2016-11-09 01:20:43 UTC |
|---|
| Accession Number | CHEM032451 |
|---|
| Identification |
|---|
| Common Name | Crocin 4 |
|---|
| Class | Small Molecule |
|---|
| Description | Crocin 4 is found in herbs and spices. Crocin 4 is isolated from saffron (Crocus sativus). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Macrocin | HMDB | | 1-Methyl 3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl (2Z,6E,8E,10Z,12E,14E)-2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptaenedioic acid | Generator |
|
|---|
| Chemical Formula | C27H36O9 |
|---|
| Average Molecular Mass | 504.569 g/mol |
|---|
| Monoisotopic Mass | 504.236 g/mol |
|---|
| CAS Registry Number | 55750-86-2 |
|---|
| IUPAC Name | 1-methyl 3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl (2Z,4E,6E,8E,10Z,12E,14E)-2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptaenedioate |
|---|
| Traditional Name | 1-methyl 3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl (2Z,4E,6E,8E,10Z,12E,14E)-2,6,11,15-tetramethylhexadeca-2,4,6,8,10,12,14-heptaenedioate |
|---|
| SMILES | COC(=O)C(\C)=C/C=C/C(/C)=C/C=C/C=C(/C)\C=C\C=C(/C)C(=O)OC1OC(CO)C(O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C27H36O9/c1-17(12-8-14-19(3)25(32)34-5)10-6-7-11-18(2)13-9-15-20(4)26(33)36-27-24(31)23(30)22(29)21(16-28)35-27/h6-15,21-24,27-31H,16H2,1-5H3/b7-6+,12-8+,13-9+,17-10+,18-11-,19-14-,20-15+ |
|---|
| InChI Key | ATQIQIBBBWQWOT-QNFREAFUSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diterpenoids. These are terpene compounds formed by four isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Diterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diterpenoid
- Hexose monosaccharide
- Fatty acid ester
- Dicarboxylic acid or derivatives
- Monosaccharide
- Oxane
- Fatty acyl
- Enoate ester
- Methyl ester
- Alpha,beta-unsaturated carboxylic ester
- Secondary alcohol
- Carboxylic acid ester
- Oxacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Polyol
- Acetal
- Organic oxygen compound
- Carbonyl group
- Primary alcohol
- Organic oxide
- Hydrocarbon derivative
- Alcohol
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0kmr-7531900000-7565f5653513f2be46ff | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-001i-2494018000-e064fa29fb8915a5806e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01r6-0439220000-02f6dda87910363f5104 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002p-1493000000-10882f143fb38a2b0aae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00m4-3490000000-6e350bc81f8318db55b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fdo-1109140000-9a390a82b3b7c34b74fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01vo-3429100000-e2e3304eefeadcafb491 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9115000000-b6f980e7433b796beb66 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0c0v-0264910000-e59b3932f081226728fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001r-0291100000-7d9c921190d33ec3c4d2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001u-3960000000-4792570a0205c3eec5da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0w29-0942270000-3363f63573993e2d23fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014l-1102900000-df8fd7adbed521054eed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05o0-2092200000-e62f336ac7337cc172d8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039122 |
|---|
| FooDB ID | FDB018632 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00054688 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 17371 |
|---|
| PubChem Compound ID | 131752554 |
|---|
| Kegg Compound ID | C00744 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|