| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:22:36 UTC |
|---|
| Update Date | 2016-11-09 01:19:28 UTC |
|---|
| Accession Number | CHEM032425 |
|---|
| Identification |
|---|
| Common Name | Calthaxanthin |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3'-Epilutein | HMDB | | (3R,3's,6'r)-Lutein | HMDB |
|
|---|
| Chemical Formula | C40H56O2 |
|---|
| Average Molecular Mass | 568.871 g/mol |
|---|
| Monoisotopic Mass | 568.428 g/mol |
|---|
| CAS Registry Number | 52842-48-5 |
|---|
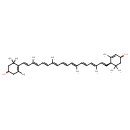
| IUPAC Name | (1R)-4-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(1R,4S)-4-hydroxy-2,6,6-trimethylcyclohex-2-en-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaen-1-yl]-3,5,5-trimethylcyclohex-3-en-1-ol |
|---|
| Traditional Name | (1R)-4-[(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(1R,4S)-4-hydroxy-2,6,6-trimethylcyclohex-2-en-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonaen-1-yl]-3,5,5-trimethylcyclohex-3-en-1-ol |
|---|
| SMILES | C\C(\C=C\C=C(/C)\C=C\C1C(C)=CC(O)CC1(C)C)=C/C=C/C=C(\C)/C=C/C=C(\C)/C=C/C1=C(C)CC(O)CC1(C)C |
|---|
| InChI Identifier | InChI=1S/C40H56O2/c1-29(17-13-19-31(3)21-23-37-33(5)25-35(41)27-39(37,7)8)15-11-12-16-30(2)18-14-20-32(4)22-24-38-34(6)26-36(42)28-40(38,9)10/h11-25,35-37,41-42H,26-28H2,1-10H3/b12-11+,17-13+,18-14+,23-21+,24-22+,29-15+,30-16+,31-19+,32-20+ |
|---|
| InChI Key | KBPHJBAIARWVSC-DKLMTRRASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthophylls. These are carotenoids containing an oxygenated carotene backbone. Carotenes are characterized by the presence of two end-groups (mostly cyclohexene rings, but also cyclopentene rings or acyclic groups) linked by a long branched alkyl chain. Carotenes belonging form a subgroup of the carotenoids family. Xanthophylls arise by oxygenation of the carotene backbone. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Tetraterpenoids |
|---|
| Direct Parent | Xanthophylls |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthophyll
- Secondary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0uk9-1000190000-79574985e26da720cbd6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-004i-3100039000-baee6b30a2822c061477 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("(3R,3'S,6'R)-b,e-Carotene-3,3'-diol,1TMS,#1" TMS) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gb9-0201190000-12a2e7baec2965c164cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0829330000-021e7fc06b639da39e2c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000b-3339330000-4e6e49a5efd708c6c971 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0000090000-cbb0d37d0ab268305e5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0000090000-d81cdc000e19ba6b5a35 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uxr-0343390000-ff8fd45055c84409ffdb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0202090000-e7ff503087b047287234 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0405490000-4908259aa27c690d5b58 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0201-0519300000-3ec7b022b5aedb2ab3d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0121930000-b93c4284d63c87f0039c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uel-0113920000-f619218ed5b32e075e70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0wmi-0059500000-ba48ee62bc6ea7e22dbf | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0003415 |
|---|
| FooDB ID | FDB112373 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00022872 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8385504 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10210008 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|