| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:21:50 UTC |
|---|
| Update Date | 2016-11-09 01:19:27 UTC |
|---|
| Accession Number | CHEM032407 |
|---|
| Identification |
|---|
| Common Name | (R)-Byakangelicin 2'-glucoside |
|---|
| Class | Small Molecule |
|---|
| Description | (R)-Byakangelicin 2'-glucoside is found in fats and oils. (R)-Byakangelicin 2'-glucoside is a constituent of angelica Angelica archangelica root. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
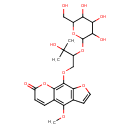
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C23H28O12 |
|---|
| Average Molecular Mass | 496.461 g/mol |
|---|
| Monoisotopic Mass | 496.158 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 9-(3-hydroxy-3-methyl-2-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}butoxy)-4-methoxy-7H-furo[3,2-g]chromen-7-one |
|---|
| Traditional Name | 9-(3-hydroxy-3-methyl-2-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}butoxy)-4-methoxyfuro[3,2-g]chromen-7-one |
|---|
| SMILES | COC1=C2C=CC(=O)OC2=C(OCC(OC2OC(CO)C(O)C(O)C2O)C(C)(C)O)C2=C1C=CO2 |
|---|
| InChI Identifier | InChI=1S/C23H28O12/c1-23(2,29)13(34-22-17(28)16(27)15(26)12(8-24)33-22)9-32-21-19-11(6-7-31-19)18(30-3)10-4-5-14(25)35-20(10)21/h4-7,12-13,15-17,22,24,26-29H,8-9H2,1-3H3 |
|---|
| InChI Key | STMLLOJFOZJAEK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 5-methoxypsoralens. These are psoralens containing a methoxy group attached at the C5 position of the psoralen group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Coumarins and derivatives |
|---|
| Sub Class | Furanocoumarins |
|---|
| Direct Parent | 5-methoxypsoralens |
|---|
| Alternative Parents | |
|---|
| Substituents | - 5-methoxypsoralen
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Benzopyran
- 1-benzopyran
- Benzofuran
- Anisole
- Alkyl aryl ether
- Pyranone
- Benzenoid
- Monosaccharide
- Pyran
- Oxane
- Heteroaromatic compound
- Furan
- Tertiary alcohol
- Secondary alcohol
- Lactone
- Organoheterocyclic compound
- Acetal
- Ether
- Oxacycle
- Polyol
- Alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Primary alcohol
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9210600000-640a7527bd8d24752491 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0a6r-9231026000-8902c058b3f27fc4256d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00n1-0129800000-bfd3a27d1c26a0ddd158 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014r-0229100000-909f969c03ddf0450bd2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0159-2397000000-229abfa5957d97e2ccb4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001j-1354900000-26514e7b99cfe5ff3223 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0292100000-f1c7ae040ac8745a3f22 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001r-2491000000-7137a7f3615797bb225c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0104900000-8fcd0820b958860d9acb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kaj-3392500000-53d1cfe63b5da3118e70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-3190000000-8247f41ae35333bc4462 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-015j-0159400000-efbf295e74ad39666273 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-1292000000-0b08179f55206290343a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-5397400000-4492fc50a72f3c2b5aff | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039070 |
|---|
| FooDB ID | FDB018569 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752535 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|