| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:21:41 UTC |
|---|
| Update Date | 2016-11-09 01:19:27 UTC |
|---|
| Accession Number | CHEM032403 |
|---|
| Identification |
|---|
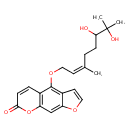
| Common Name | 4-[(6,7-Dihydroxy-3,7-dimethyl-2-octenyl)oxy]-7H-furo[3,2-g][1]benzopyran-7-one |
|---|
| Class | Small Molecule |
|---|
| Description | 6',7'-Dihydroxybergamottin is found in citrus. 6',7'-Dihydroxybergamottin is isolated from Citrus macroptera whole fruits, a non-commercial sp. of the South Pacific. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-[(6,7-Dihydroxy-3,7-dimethyl-2-octenyl)oxy]-7H-furo[3,2-g][1]benzopyran-7-one | HMDB | | Lepiochlorin | HMDB | | 6,7-DHB | MeSH | | 6',7'-Dihydroxybergamottin | MeSH |
|
|---|
| Chemical Formula | C21H24O6 |
|---|
| Average Molecular Mass | 372.417 g/mol |
|---|
| Monoisotopic Mass | 372.157 g/mol |
|---|
| CAS Registry Number | 71339-34-9 |
|---|
| IUPAC Name | 4-{[(2Z)-6,7-dihydroxy-3,7-dimethyloct-2-en-1-yl]oxy}-7H-furo[3,2-g]chromen-7-one |
|---|
| Traditional Name | 4-{[(2Z)-6,7-dihydroxy-3,7-dimethyloct-2-en-1-yl]oxy}furo[3,2-g]chromen-7-one |
|---|
| SMILES | C\C(CCC(O)C(C)(C)O)=C\COC1=C2C=COC2=CC2=C1C=CC(=O)O2 |
|---|
| InChI Identifier | InChI=1S/C21H24O6/c1-13(4-6-18(22)21(2,3)24)8-10-26-20-14-5-7-19(23)27-17(14)12-16-15(20)9-11-25-16/h5,7-9,11-12,18,22,24H,4,6,10H2,1-3H3/b13-8- |
|---|
| InChI Key | IXZUPBUEKFXTSD-JYRVWZFOSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as psoralens. These are organic compounds containing a psoralen moiety, which consists of a furan fused to a chromenone to for 7H-furo[3,2-g]chromen-7-one. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Coumarins and derivatives |
|---|
| Sub Class | Furanocoumarins |
|---|
| Direct Parent | Psoralens |
|---|
| Alternative Parents | |
|---|
| Substituents | - Psoralen
- Benzopyran
- 1-benzopyran
- Benzofuran
- Fatty alcohol
- Alkyl aryl ether
- Pyranone
- Fatty acyl
- Benzenoid
- Pyran
- Heteroaromatic compound
- Tertiary alcohol
- Furan
- 1,2-diol
- Secondary alcohol
- Lactone
- Oxacycle
- Ether
- Organoheterocyclic compound
- Organic oxygen compound
- Hydrocarbon derivative
- Alcohol
- Organic oxide
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9044000000-941ed62632b71a13305d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0kji-8894750000-911d6c503b24adefc3d3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-0219000000-60828bda7a2eb1b2b838 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zni-6898000000-9b3d898ebda6aef35d94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ktr-9230000000-58a1d646ec73e9fb6806 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fk9-0029000000-739b06d1a0a3b5242e1b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-0395000000-0b1e14b4c9f49fd15767 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-2920000000-580ae694ecab81deb640 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f79-0029000000-53d804be2c67f474ea3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f79-1159000000-3f605d41a0289f2dbcba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kdr-7892000000-2bf483e2025be4fe392a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0019000000-492869cd0ced7b279ef4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0pb9-9188000000-c36742fd1abb17e61818 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zfr-2980000000-541760eb3e74dd26805c | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039066 |
|---|
| FooDB ID | FDB018565 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00047681 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | 6',7'-Dihydroxybergamottin |
|---|
| Chemspider ID | 35014735 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752532 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|