| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:21:20 UTC |
|---|
| Update Date | 2016-11-09 01:19:27 UTC |
|---|
| Accession Number | CHEM032394 |
|---|
| Identification |
|---|
| Common Name | 9-Hydroxy-4-(3,7-dimethyl-2,6-octadienyloxy)-psoralen |
|---|
| Class | Small Molecule |
|---|
| Description | 9-Hydroxy-4-(3,7-dimethyl-2,6-octadienyloxy)-psoralen is found in citrus. 9-Hydroxy-4-(3,7-dimethyl-2,6-octadienyloxy)-psoralen is a constituent of Citrus medica (citron). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
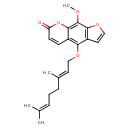
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Protorifamycin I | HMDB |
|
|---|
| Chemical Formula | C22H24O5 |
|---|
| Average Molecular Mass | 368.423 g/mol |
|---|
| Monoisotopic Mass | 368.162 g/mol |
|---|
| CAS Registry Number | 69239-53-8 |
|---|
| IUPAC Name | 4-{[(2E)-3,7-dimethylocta-2,6-dien-1-yl]oxy}-9-methoxy-7H-furo[3,2-g]chromen-7-one |
|---|
| Traditional Name | 4-{[(2E)-3,7-dimethylocta-2,6-dien-1-yl]oxy}-9-methoxyfuro[3,2-g]chromen-7-one |
|---|
| SMILES | COC1=C2OC(=O)C=CC2=C(OC\C=C(/C)CCC=C(C)C)C2=C1OC=C2 |
|---|
| InChI Identifier | InChI=1S/C22H24O5/c1-14(2)6-5-7-15(3)10-12-25-19-16-8-9-18(23)27-21(16)22(24-4)20-17(19)11-13-26-20/h6,8-11,13H,5,7,12H2,1-4H3/b15-10+ |
|---|
| InChI Key | OQHQALGVQDTJDN-XNTDXEJSSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 8-methoxypsoralens. These are psoralens containing a methoxy group attached at the C8 position of the psoralen group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Coumarins and derivatives |
|---|
| Sub Class | Furanocoumarins |
|---|
| Direct Parent | 8-methoxypsoralens |
|---|
| Alternative Parents | |
|---|
| Substituents | - 8-methoxypsoralen
- Terpene lactone
- Aromatic monoterpenoid
- Benzopyran
- Monoterpenoid
- 1-benzopyran
- Benzofuran
- Anisole
- Alkyl aryl ether
- Pyranone
- Benzenoid
- Pyran
- Furan
- Heteroaromatic compound
- Lactone
- Ether
- Organoheterocyclic compound
- Oxacycle
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ukl-6479000000-c570fee77df7a705ca90 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0319000000-0d729bc45ebd424287ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-067r-7956000000-9dd9639b04a764433fa6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-9231000000-8852b3c18c530af8f13c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0029000000-acf1520a0d3e19084f97 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01q9-0294000000-0bd6f5a975b7d768015d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01p9-1970000000-43a48095b683bcd8545d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-7b7777091d83071e8781 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01c0-0395000000-9a20284d011cc2c8affd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0j4r-3490000000-19917ec649e2db5844eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-f8867cbe344d3e6bacad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-6090000000-9c93ca565dbaaf7ecfc2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9170000000-9e4d88d23771b43a3309 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039057 |
|---|
| FooDB ID | FDB018555 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4944685 |
|---|
| ChEBI ID | 174907 |
|---|
| PubChem Compound ID | 6440422 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|