| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:20:45 UTC |
|---|
| Update Date | 2016-11-09 01:19:27 UTC |
|---|
| Accession Number | CHEM032380 |
|---|
| Identification |
|---|
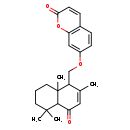
| Common Name | (3'x,5'a,9'x,10'b)-O-(6-Oxo-7-drimen-11-yl)umbelliferone |
|---|
| Class | Small Molecule |
|---|
| Description | (3'x,5'a,9'x,10'b)-O-(6-Oxo-7-drimen-11-yl)umbelliferone is found in herbs and spices. (3'x,5'a,9'x,10'b)-O-(6-Oxo-7-drimen-11-yl)umbelliferone is a constituent of Ferula galbaniflua (galbanum). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C24H28O4 |
|---|
| Average Molecular Mass | 380.477 g/mol |
|---|
| Monoisotopic Mass | 380.199 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 7-[(2,5,5,8a-tetramethyl-4-oxo-1,4,4a,5,6,7,8,8a-octahydronaphthalen-1-yl)methoxy]-2H-chromen-2-one |
|---|
| Traditional Name | 7-[(2,5,5,8a-tetramethyl-4-oxo-4a,6,7,8-tetrahydro-1H-naphthalen-1-yl)methoxy]chromen-2-one |
|---|
| SMILES | CC1=CC(=O)C2C(C)(C)CCCC2(C)C1COC1=CC2=C(C=CC(=O)O2)C=C1 |
|---|
| InChI Identifier | InChI=1S/C24H28O4/c1-15-12-19(25)22-23(2,3)10-5-11-24(22,4)18(15)14-27-17-8-6-16-7-9-21(26)28-20(16)13-17/h6-9,12-13,18,22H,5,10-11,14H2,1-4H3 |
|---|
| InChI Key | CKZQOSCRZFMJSZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as coumarins and derivatives. These are polycyclic aromatic compounds containing a 1-benzopyran moiety with a ketone group at the C2 carbon atom (1-benzopyran-2-one). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Coumarins and derivatives |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Coumarins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coumarin
- Benzopyran
- 1-benzopyran
- Alkyl aryl ether
- Cyclohexenone
- Pyranone
- Pyran
- Benzenoid
- Heteroaromatic compound
- Lactone
- Ketone
- Oxacycle
- Ether
- Organoheterocyclic compound
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0gi0-3519000000-c8d8cd5207f40a21968e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0039000000-cffb68c3721cfd0582ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03yi-4459000000-5dc7110f1545aad5b9bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02pl-6910000000-e6bda90ea54b523b97f9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0309000000-cc2b4668c842f5880417 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fr-0609000000-54ef359ab41f8ece8bed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-1900000000-53ba6d96bb82c963d453 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0009000000-27af3493aa773bcf1b77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fr-0729000000-066d484cd70a51a538c1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0910000000-bea1b4019163ad7905b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03yi-0649000000-f663d025c3fa6c2ff018 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03fr-1839000000-121f45a00e344486eec4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-07vr-4921000000-d367b9faefc7d114dedf | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039043 |
|---|
| FooDB ID | FDB018540 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014727 |
|---|
| ChEBI ID | 172030 |
|---|
| PubChem Compound ID | 131752525 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|