| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:19:40 UTC |
|---|
| Update Date | 2016-11-09 01:19:27 UTC |
|---|
| Accession Number | CHEM032350 |
|---|
| Identification |
|---|
| Common Name | 15beta,19-ent-Dihydroxy-7-trachylobanone |
|---|
| Class | Small Molecule |
|---|
| Description | Constituent of Helianthus annuus (sunflower). 15beta,19-ent-Dihydroxy-7-trachylobanone is found in fats and oils. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
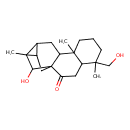
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (ent-15b)-15,19-Dihydroxy-7-trachylobanone | Generator | | (ent-15Β)-15,19-dihydroxy-7-trachylobanone | Generator | | 2,6-Dimenapqi | HMDB | | 2,6-Dimethyl-N-acetyl-p-benzoquinoneimine | HMDB | | 2,6-Dimethyl-NAPQI | HMDB | | Acetyl-2,6-dimethyl-4-benzoquinone imine | HMDB | | Acetyl-2,6-dimethyl-p-benzoquinoneimine | HMDB | | Na-2,6-bqi | HMDB |
|
|---|
| Chemical Formula | C20H30O3 |
|---|
| Average Molecular Mass | 318.450 g/mol |
|---|
| Monoisotopic Mass | 318.219 g/mol |
|---|
| CAS Registry Number | 137648-01-2 |
|---|
| IUPAC Name | 16-hydroxy-5-(hydroxymethyl)-5,9,13-trimethylpentacyclo[11.2.1.0¹,¹⁰.0⁴,⁹.0¹²,¹⁴]hexadecan-2-one |
|---|
| Traditional Name | 16-hydroxy-5-(hydroxymethyl)-5,9,13-trimethylpentacyclo[11.2.1.0¹,¹⁰.0⁴,⁹.0¹²,¹⁴]hexadecan-2-one |
|---|
| SMILES | CC12C3CC4(C1O)C(CC23)C1(C)CCCC(C)(CO)C1CC4=O |
|---|
| InChI Identifier | InChI=1S/C20H30O3/c1-17(10-21)5-4-6-18(2)13(17)8-15(22)20-9-12-11(7-14(18)20)19(12,3)16(20)23/h11-14,16,21,23H,4-10H2,1-3H3 |
|---|
| InChI Key | SSTASBUCOHAYRJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as kaurane diterpenoids. These are diterpene alkaloids with a structure that is based on the kaurane skeleton. Kaurane is a tetracyclic compound that arises by cyclisation of a pimarane precursor followed by rearrangement. It possesses a [3,2,1]-bicyclic ring system with C15-C16 bridge connected to C13, forming the five-membered ring D. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Diterpenoids |
|---|
| Direct Parent | Kaurane diterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Kaurane diterpenoid
- Villanovane, atisane, trachylobane or helvifulvane diterpenoid
- Atisane diterpenoid
- Alkaloid or derivatives
- Cyclic alcohol
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ue9-1963000000-ddd3b7d8bac97a724563 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01xt-5182900000-9cf9694abe19031e84e0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0uxr-0039000000-eaa2e87c7455613325b7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-1297000000-55ed954fd3c32eab8c59 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00l6-6490000000-9876d514ec6d96f259a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0059000000-645fc2c89b4f5f1876d0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-0094000000-c6fc0b0d0b443daf1299 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-5191000000-4f4ee0c12aa6027b03ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-062ad2a35e11d303cf23 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0009000000-062ad2a35e11d303cf23 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0019000000-ab03c918928dcda96131 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gbi-0049000000-38b89b1c6158c570ac62 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gbi-0955000000-a020c69ed7aef93ee58f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0596-3900000000-e9bc4240346f8bc63752 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0039012 |
|---|
| FooDB ID | FDB018506 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752509 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|