| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:18:15 UTC |
|---|
| Update Date | 2016-11-09 01:19:26 UTC |
|---|
| Accession Number | CHEM032316 |
|---|
| Identification |
|---|
| Common Name | (1beta,2beta,5beta)-p-Menth-3-ene-1,2,5-triol |
|---|
| Class | Small Molecule |
|---|
| Description | (1beta,2beta,5beta)-p-Menth-3-ene-1,2,5-triol is a constituent of Carum ajowan (ajowan). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
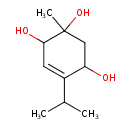
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (1b,2b,5b)-p-Menth-3-ene-1,2,5-triol | Generator | | (1Β,2β,5β)-p-menth-3-ene-1,2,5-triol | Generator |
|
|---|
| Chemical Formula | C10H18O3 |
|---|
| Average Molecular Mass | 186.248 g/mol |
|---|
| Monoisotopic Mass | 186.126 g/mol |
|---|
| CAS Registry Number | 359875-80-2 |
|---|
| IUPAC Name | 2-methyl-5-(propan-2-yl)cyclohex-5-ene-1,2,4-triol |
|---|
| Traditional Name | 5-isopropyl-2-methylcyclohex-5-ene-1,2,4-triol |
|---|
| SMILES | CC(C)C1=CC(O)C(C)(O)CC1O |
|---|
| InChI Identifier | InChI=1S/C10H18O3/c1-6(2)7-4-9(12)10(3,13)5-8(7)11/h4,6,8-9,11-13H,5H2,1-3H3 |
|---|
| InChI Key | SYRVWXFZYGDVTF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as menthane monoterpenoids. These are monoterpenoids with a structure based on the o-, m-, or p-menthane backbone. P-menthane consists of the cyclohexane ring with a methyl group and a (2-methyl)-propyl group at the 1 and 4 ring position, respectively. The o- and m- menthanes are much rarer, and presumably arise by alkyl migration of p-menthanes. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Monoterpenoids |
|---|
| Direct Parent | Menthane monoterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - P-menthane monoterpenoid
- Monocyclic monoterpenoid
- Cyclitol or derivatives
- Tertiary alcohol
- Secondary alcohol
- Polyol
- Organic oxygen compound
- Hydrocarbon derivative
- Organooxygen compound
- Alcohol
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00kg-9600000000-eea2432f99dd2fee0437 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-002r-5219000000-678b458dde2906c1f2ea | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0900000000-8fbcb907216b947a533e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-3900000000-c3958663ef7a2240af39 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-9400000000-a4687f8e4bd38aa32dae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-f2508e59d0be57126531 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00kr-3900000000-4ba2425ce4d82fc4728d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014j-9500000000-98b928e3bac8eefd6170 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1900000000-5ed80d2c977c8fa34439 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0frb-9500000000-fc1fbb081d9f1c3456e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kf-9000000000-0e0a2ef28aada82054e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-822190e00cd01cf01d8e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0900000000-21653be9756962aa99e7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006y-9200000000-e19f19ef01f3b21896f4 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038978 |
|---|
| FooDB ID | FDB018464 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014706 |
|---|
| ChEBI ID | 138751 |
|---|
| PubChem Compound ID | 85408483 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|