| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:12:35 UTC |
|---|
| Update Date | 2016-11-09 01:19:25 UTC |
|---|
| Accession Number | CHEM032197 |
|---|
| Identification |
|---|
| Common Name | 3-O-alpha-L-Arabinopyranosyl-L-arabinose |
|---|
| Class | Small Molecule |
|---|
| Description | Hydrolytic product of the mucilage from the stems of Opuntia ficus-indica (Indian fig). 3-O-alpha-L-Arabinopyranosyl-L-arabinose is found in fruits. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
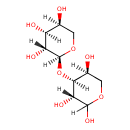
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-O-a-L-Arabinopyranosyl-L-arabinose | Generator | | 3-O-Α-L-arabinopyranosyl-L-arabinose | Generator |
|
|---|
| Chemical Formula | C10H18O9 |
|---|
| Average Molecular Mass | 282.245 g/mol |
|---|
| Monoisotopic Mass | 282.095 g/mol |
|---|
| CAS Registry Number | 79435-28-2 |
|---|
| IUPAC Name | (2S,3R,4S,5S)-2-{[(3R,4S,5S)-2,3,5-trihydroxyoxan-4-yl]oxy}oxane-3,4,5-triol |
|---|
| Traditional Name | (2S,3R,4S,5S)-2-{[(3R,4S,5S)-2,3,5-trihydroxyoxan-4-yl]oxy}oxane-3,4,5-triol |
|---|
| SMILES | O[C@H]1CO[C@@H](O[C@H]2[C@@H](O)COC(O)[C@@H]2O)[C@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C10H18O9/c11-3-1-18-10(6(14)5(3)13)19-8-4(12)2-17-9(16)7(8)15/h3-16H,1-2H2/t3-,4-,5-,6+,7+,8-,9?,10-/m0/s1 |
|---|
| InChI Key | FVPQAMUWCNJRQW-XTZOYHFESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as o-glycosyl compounds. These are glycoside in which a sugar group is bonded through one carbon to another group via a O-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | O-glycosyl compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - O-glycosyl compound
- Disaccharide
- Oxane
- Secondary alcohol
- Hemiacetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Acetal
- Hydrocarbon derivative
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00lr-0490000000-58d396d2d0abdb619126 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00lr-0950000000-a6defa13680b0e88a58e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fz9-8910000000-741a37a11e228626248c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-1290000000-ca740044f4ed4e06f23c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01qa-2950000000-61f0446990b7e5188a42 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-59517e35a27fadf06fc3 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | FDB018294 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|