| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:12:01 UTC |
|---|
| Update Date | 2016-11-09 01:19:25 UTC |
|---|
| Accession Number | CHEM032183 |
|---|
| Identification |
|---|
| Common Name | 1-(m-Methoxycinnamoyl)pyrrolidine |
|---|
| Class | Small Molecule |
|---|
| Description | 1-(m-Methoxycinnamoyl)pyrrolidine is found in beverages. 1-(m-Methoxycinnamoyl)pyrrolidine is an alkaloid from the roots of kava (Piper methysticum). FDA advises against use of kava in food due to potential risk of severe liver damage (2002). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
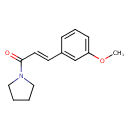
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(m-Methoxycinnamoyl)pyrrolidine, 8ci | HMDB | | m-Methoxycinnamic acid pyrrolidide | HMDB |
|
|---|
| Chemical Formula | C14H17NO2 |
|---|
| Average Molecular Mass | 231.290 g/mol |
|---|
| Monoisotopic Mass | 231.126 g/mol |
|---|
| CAS Registry Number | 29647-01-6 |
|---|
| IUPAC Name | (2E)-3-(3-methoxyphenyl)-1-(pyrrolidin-1-yl)prop-2-en-1-one |
|---|
| Traditional Name | (2E)-3-(3-methoxyphenyl)-1-(pyrrolidin-1-yl)prop-2-en-1-one |
|---|
| SMILES | COC1=CC=CC(\C=C\C(=O)N2CCCC2)=C1 |
|---|
| InChI Identifier | InChI=1S/C14H17NO2/c1-17-13-6-4-5-12(11-13)7-8-14(16)15-9-2-3-10-15/h4-8,11H,2-3,9-10H2,1H3/b8-7+ |
|---|
| InChI Key | LAPTWLCIZWFMJK-BQYQJAHWSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as coumaric acids and derivatives. These are aromatic compounds containing Aromatic compounds containing a cinnamic acid moiety (or a derivative thereof) hydroxylated at the C2 (ortho-), C3 (meta-), or C4 (para-) carbon atom of the benzene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Cinnamic acids and derivatives |
|---|
| Sub Class | Hydroxycinnamic acids and derivatives |
|---|
| Direct Parent | Coumaric acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Coumaric acid or derivatives
- Phenoxy compound
- Anisole
- N-acylpyrrolidine
- Methoxybenzene
- Phenol ether
- Styrene
- Alkyl aryl ether
- Monocyclic benzene moiety
- Benzenoid
- Tertiary carboxylic acid amide
- Pyrrolidine
- Carboxamide group
- Carboxylic acid derivative
- Ether
- Azacycle
- Organoheterocyclic compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic nitrogen compound
- Organic oxide
- Carbonyl group
- Organonitrogen compound
- Organooxygen compound
- Organopnictogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0hr0-9870000000-608b01bc89f74a539e7f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-2190000000-ee659fae3071a622b221 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0089-7950000000-50f418a9718405d92d26 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fu-9400000000-fad8489909ac4e06ea48 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-c287c7982eed044607c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0089-7490000000-b4d9ea391271098ced16 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9300000000-56b5bb3c03841207cb6f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0590000000-398492c34cfbe2f26908 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0089-4940000000-50473043a7da2f7d1827 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-6910000000-e973adc46ec09cd91ee1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-219884484b7e41d6161e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0159-2950000000-c57baf9fb68f6647dc77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01b9-0900000000-75795fe4187dcffc54c1 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038839 |
|---|
| FooDB ID | FDB018274 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4523676 |
|---|
| ChEBI ID | 157735 |
|---|
| PubChem Compound ID | 5373710 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|