| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:11:43 UTC |
|---|
| Update Date | 2016-11-09 01:19:25 UTC |
|---|
| Accession Number | CHEM032176 |
|---|
| Identification |
|---|
| Common Name | 2,9-Dimethyl-2,9-diazatricyclo[10.2.2.25,8]octadeca-5,7,12,14,15,17-hexaene-3,10-diol, 9CI |
|---|
| Class | Small Molecule |
|---|
| Description | Alkaloid from peelings of Citrus unshiu (satsuma mandarin). 2,9-Dimethyl-2,9-diazatricyclo[10.2.2.25,8]octadeca-5,7,12,14,15,17-hexaene-3,10-diol, 9CI is found in citrus. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
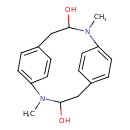
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| N,A',n',a-cyclodi(4-methylaminobenzeneethanol) | HMDB |
|
|---|
| Chemical Formula | C18H22N2O2 |
|---|
| Average Molecular Mass | 298.380 g/mol |
|---|
| Monoisotopic Mass | 298.168 g/mol |
|---|
| CAS Registry Number | 124190-18-7 |
|---|
| IUPAC Name | 2,9-dimethyl-2,9-diazatricyclo[10.2.2.2⁵,⁸]octadeca-1(14),5,7,12,15,17-hexaene-3,10-diol |
|---|
| Traditional Name | 2,9-dimethyl-2,9-diazatricyclo[10.2.2.2⁵,⁸]octadeca-1(14),5,7,12,15,17-hexaene-3,10-diol |
|---|
| SMILES | CN1C(O)CC2=CC=C(C=C2)N(C)C(O)CC2=CC=C1C=C2 |
|---|
| InChI Identifier | InChI=1S/C18H22N2O2/c1-19-15-7-3-14(4-8-15)12-18(22)20(2)16-9-5-13(6-10-16)11-17(19)21/h3-10,17-18,21-22H,11-12H2,1-2H3 |
|---|
| InChI Key | ZKYBZOKMKHPBAJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dialkylarylamines. These are aliphatic aromatic amines in which the amino group is linked to two aliphatic chains and one aromatic group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | Dialkylarylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dialkylarylamine
- Benzenoid
- Azacycle
- Organoheterocyclic compound
- Alkanolamine
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001j-0090000000-3feb780734f95b79af09 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0fmi-9026300000-1997fe56cfc124d6a38e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-3783e06a7a481a5f6608 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0090000000-b58a57a73d091a90ee74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-0090000000-0a212ec23c8a4935869f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-bb2486640ee7d3619831 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-06f80890df9e7892bf37 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00l2-0090000000-4ee3bbd215348a8f82a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-1ff8a80a90c015635a67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0090000000-99942e497afc20914003 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-0090000000-81a145da8d8120fc43d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-98af63196478b2a14f8a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-1ecdd275e225fcd1491c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ta-0090000000-512ab542d3941f6822dd | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038832 |
|---|
| FooDB ID | FDB018266 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752475 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|