| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:10:10 UTC |
|---|
| Update Date | 2016-11-09 01:19:24 UTC |
|---|
| Accession Number | CHEM032139 |
|---|
| Identification |
|---|
| Common Name | 7,9-Illudadiene-3,14-diol |
|---|
| Class | Small Molecule |
|---|
| Description | 7,9-Illudadiene-3,14-diol is found in mushrooms. 7,9-Illudadiene-3,14-diol is a metabolite of Agrocybe aegerita. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
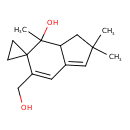
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C15H22O2 |
|---|
| Average Molecular Mass | 234.334 g/mol |
|---|
| Monoisotopic Mass | 234.162 g/mol |
|---|
| CAS Registry Number | 141940-51-4 |
|---|
| IUPAC Name | 6'-(hydroxymethyl)-2',2',4'-trimethyl-2',3',3'a,4'-tetrahydrospiro[cyclopropane-1,5'-indene]-4'-ol |
|---|
| Traditional Name | 6'-(hydroxymethyl)-2',2',4'-trimethyl-3',3'a-dihydrospiro[cyclopropane-1,5'-indene]-4'-ol |
|---|
| SMILES | CC1(C)CC2C(=C1)C=C(CO)C1(CC1)C2(C)O |
|---|
| InChI Identifier | InChI=1S/C15H22O2/c1-13(2)7-10-6-11(9-16)15(4-5-15)14(3,17)12(10)8-13/h6-7,12,16-17H,4-5,8-9H2,1-3H3 |
|---|
| InChI Key | YEDYYAKSTVQMBN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as illudanes and illudins. These are sesquiterpenoids containing either the illudane moiety (based on a 3,6,6,7b-tetramethyl-decahydro-1H-cyclobuta[e]indene ring system), the illudin moiety (2',2',4',6'-tetramethyl-octahydrospiro[cyclopropane-1,5'-indene]), or a derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Illudanes and illudins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Illudine sesquiterpenoid
- Tertiary alcohol
- Organic oxygen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0gdr-3930000000-24fc050a6ee707d9440f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-03fr-7098000000-2d6285a16ec547b2fc08 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-1390000000-b87bdc0c97106f36486b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014s-8980000000-e4830dce9c13b79b96bb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000l-9300000000-675bbb7d48aeaa02ee69 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-9b5384fb8d0d731f6247 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fsi-0290000000-cd85e5ec7f9015c6dfca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-7940000000-19558cef7a5bbdb6b07c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-ee56b8a065eb4a8064ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0190000000-721cbc0fe5ed355a490f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001r-2690000000-f29d0f68707ff4404274 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0190000000-7deea30f35d37a528c2b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-1590000000-6b9fb33493da156ed8e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000l-9270000000-c722673261c8856fcc4b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038795 |
|---|
| FooDB ID | FDB018220 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014666 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 91752244 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|