| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:09:34 UTC |
|---|
| Update Date | 2016-11-09 01:19:24 UTC |
|---|
| Accession Number | CHEM032126 |
|---|
| Identification |
|---|
| Common Name | 7-Hydroxy-2',4',5'-trimethoxyisoflavan |
|---|
| Class | Small Molecule |
|---|
| Description | 7-Hydroxy-2',4',5'-trimethoxyisoflavan is found in alfalfa. 7-Hydroxy-2',4',5'-trimethoxyisoflavan is isolated from the leaves of Medicago sativa (alfalfa). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
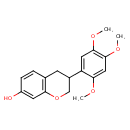
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5'-Methoxysativan | HMDB |
|
|---|
| Chemical Formula | C18H20O5 |
|---|
| Average Molecular Mass | 316.348 g/mol |
|---|
| Monoisotopic Mass | 316.131 g/mol |
|---|
| CAS Registry Number | 122577-99-5 |
|---|
| IUPAC Name | 3-(2,4,5-trimethoxyphenyl)-3,4-dihydro-2H-1-benzopyran-7-ol |
|---|
| Traditional Name | 3-(2,4,5-trimethoxyphenyl)-3,4-dihydro-2H-1-benzopyran-7-ol |
|---|
| SMILES | COC1=CC(OC)=C(OC)C=C1C1COC2=C(C1)C=CC(O)=C2 |
|---|
| InChI Identifier | InChI=1S/C18H20O5/c1-20-16-9-18(22-3)17(21-2)8-14(16)12-6-11-4-5-13(19)7-15(11)23-10-12/h4-5,7-9,12,19H,6,10H2,1-3H3 |
|---|
| InChI Key | RXXVCVOYIGXBFH-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 4'-o-methylated isoflavonoids. These are isoflavonoids with methoxy groups attached to the C4' atom of the isoflavonoid backbone. Isoflavonoids are natural products derived from 3-phenylchromen-4-one. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Isoflavonoids |
|---|
| Sub Class | O-methylated isoflavonoids |
|---|
| Direct Parent | 4'-O-methylated isoflavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 4p-methoxyisoflavonoid
- 2p-methoxyisoflavonoid-skeleton
- 3p-methoxyisoflavonoid-skeleton
- Isoflavanol
- Hydroxyisoflavonoid
- Isoflavan
- 1-benzopyran
- Chromane
- Benzopyran
- Methoxybenzene
- Phenol ether
- Anisole
- Phenoxy compound
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Monocyclic benzene moiety
- Oxacycle
- Organoheterocyclic compound
- Ether
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udr-0595000000-9d33ef883f4183c7403c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-2319000000-3edc8a22bcf55c7025d5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01b9-0928000000-0a789da96f2aa92e879d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0934000000-dd4ac001524602ad316a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05fr-1920000000-e36bdbfc7cd1ed34e77e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0319000000-a58bc85322ef49641022 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-066r-0793000000-cbf55111aa57178f8685 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-082i-2980000000-2e69e1cb1025830ab6ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0409000000-376638483220aca64b1e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0739000000-876d39bcb08afe60746a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ea-0920000000-12898d08a52d90a767ff | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0009000000-23ac50f9ae12ab6825c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-0297000000-cbf302648f7c34865ae9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0229-2963000000-360391315bdfadeb4d66 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038780 |
|---|
| FooDB ID | FDB018199 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00020173 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014658 |
|---|
| ChEBI ID | 175068 |
|---|
| PubChem Compound ID | 14394138 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|