| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:08:28 UTC |
|---|
| Update Date | 2016-11-09 01:19:24 UTC |
|---|
| Accession Number | CHEM032101 |
|---|
| Identification |
|---|
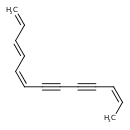
| Common Name | (E,E,E)-1,3,5,11-Tridecatetraene-7,9-diyne |
|---|
| Class | Small Molecule |
|---|
| Description | (3Z,5E,11E)-1,3,5,11-Tridecatetraene-7,9-diyne is found in fats and oils. (3Z,5E,11E)-1,3,5,11-Tridecatetraene-7,9-diyne is isolated from Carthamus tinctorius (safflower). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (e,e,e)-1,3,5,11-Tridecatriene-7,9-diyne | HMDB | | 1,3(e),5(e),11(e)-Tridecatetraen-7,9-diyne | HMDB | | 1,3,5,11-Tridecatetraene-7,9-diyne | HMDB | | 1,3,5,11-Tridecatetraene-7,9-diyne isomer # 2 | HMDB | | 1,3,5,7-Tridecatetraene-7,9-diyne isomer # 1 | HMDB | | 3,6-Bis[trichloromethyl]pyridazine | HMDB | | 5-[P-chlorobenzylidenamino]-2,4-Dichlorobenzoic acid | HMDB |
|
|---|
| Chemical Formula | C13H12 |
|---|
| Average Molecular Mass | 168.234 g/mol |
|---|
| Monoisotopic Mass | 168.094 g/mol |
|---|
| CAS Registry Number | 17091-00-8 |
|---|
| IUPAC Name | (3E,5Z,11Z)-trideca-1,3,5,11-tetraen-7,9-diyne |
|---|
| Traditional Name | (3E,5Z,11Z)-trideca-1,3,5,11-tetraen-7,9-diyne |
|---|
| SMILES | C\C=C/C#CC#C\C=C/C=C/C=C |
|---|
| InChI Identifier | InChI=1S/C13H12/c1-3-5-7-9-11-13-12-10-8-6-4-2/h3-7,9,11H,1H2,2H3/b6-4-,7-5+,11-9- |
|---|
| InChI Key | ASVIELUINMCNMW-SZFGQAOXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as enynes. These are hydrocarbons containing an alkene and an alkyne group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Hydrocarbons |
|---|
| Class | Unsaturated hydrocarbons |
|---|
| Sub Class | Enynes |
|---|
| Direct Parent | Enynes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Enyne
- Unsaturated aliphatic hydrocarbon
- Olefin
- Acyclic olefin
- Acyclic acetylene
- Acetylene
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0gbc-5900000000-4ddec32acd7b949ce0b1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-1900000000-ec95b1259274dc916898 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-5900000000-0532785e1499262fbd9a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-9200000000-a314a28096aea3410c77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-5281d390e6939c6071c9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0900000000-a6a1402dac4248e7de9b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uxr-8900000000-51c25fa7fe7cd935b6ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0900000000-05aecce78fb6c7de11ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0900000000-a4bf2ac6d945ee06954f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01p9-7900000000-420cf65b5669f0dd5bed | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-7900000000-d2c6080941ed8edd64e0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ftu-9400000000-578888109222520363df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fb9-9200000000-fad45cf90ee34ad133c1 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038754 |
|---|
| FooDB ID | FDB018168 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30777290 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752453 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|