| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:08:23 UTC |
|---|
| Update Date | 2016-11-09 01:19:24 UTC |
|---|
| Accession Number | CHEM032099 |
|---|
| Identification |
|---|
| Common Name | Distichonic acid A |
|---|
| Class | Small Molecule |
|---|
| Description | Distichonic acid A is found in cereals and cereal products. Distichonic acid A is produced by Hordeum vulgare (barley). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
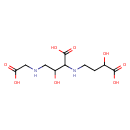
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Distichonate a | Generator | | 2'-Distichonic acid a | HMDB | | 2'-Epi-distichonic acid a | HMDB | | N-(3-Carboxy-3-hydroxypropyl)-4-[(carboxymethyl)amino]-L-allothreonine | HMDB | | 2-[(3-Carboxy-3-hydroxypropyl)amino]-4-[(carboxymethyl)amino]-3-hydroxybutanoate | Generator |
|
|---|
| Chemical Formula | C10H18N2O8 |
|---|
| Average Molecular Mass | 294.259 g/mol |
|---|
| Monoisotopic Mass | 294.106 g/mol |
|---|
| CAS Registry Number | 84495-20-5 |
|---|
| IUPAC Name | 2-[(3-carboxy-3-hydroxypropyl)amino]-4-[(carboxymethyl)amino]-3-hydroxybutanoic acid |
|---|
| Traditional Name | 2-[(3-carboxy-3-hydroxypropyl)amino]-4-[(carboxymethyl)amino]-3-hydroxybutanoic acid |
|---|
| SMILES | OC(CNCC(O)=O)C(NCCC(O)C(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C10H18N2O8/c13-5(9(17)18)1-2-12-8(10(19)20)6(14)3-11-4-7(15)16/h5-6,8,11-14H,1-4H2,(H,15,16)(H,17,18)(H,19,20) |
|---|
| InChI Key | SEATYFZPTMHEIW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gamma amino acids and derivatives. These are amino acids having a (-NH2) group attached to the gamma carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Gamma amino acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Gamma amino acid or derivatives
- Alpha-amino acid
- Alpha-amino acid or derivatives
- Tricarboxylic acid or derivatives
- Amino fatty acid
- Beta-hydroxy acid
- Hydroxy fatty acid
- Alpha-hydroxy acid
- Hydroxy acid
- Monosaccharide
- Fatty acyl
- 1,3-aminoalcohol
- Secondary alcohol
- 1,2-aminoalcohol
- Amino acid
- Secondary amine
- Secondary aliphatic amine
- Carboxylic acid
- Organonitrogen compound
- Carbonyl group
- Organic oxide
- Organopnictogen compound
- Amine
- Hydrocarbon derivative
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002f-9680000000-2068a70375ca26f8bb80 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-000l-2121497000-79c5ece41ead7478df81 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-057j-0090000000-1ce37fb09fb1cdca3c25 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-0290000000-0849afc530d115af1c01 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f89-7790000000-bbe839b9679acb1659b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0035-0290000000-44dd29cd45556ef5b88a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f8a-2390000000-7b71ec1d6d9a6f6239ba | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-9830000000-477bb88985d562a6923e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0090000000-84671dbaab8714bd2bfe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004j-1390000000-286c354623bea881ab55 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05g0-9710000000-47f2fb30bec114903880 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0290000000-7fac7abff4cac9148700 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kcr-1920000000-87c4c48432eac85a6e13 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00xr-9810000000-f0126aa30c41080d629d | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038752 |
|---|
| FooDB ID | FDB018164 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00054661 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014653 |
|---|
| ChEBI ID | 168140 |
|---|
| PubChem Compound ID | 85405750 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|