| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:06:17 UTC |
|---|
| Update Date | 2016-11-09 01:19:23 UTC |
|---|
| Accession Number | CHEM032045 |
|---|
| Identification |
|---|
| Common Name | Oryzalic acid B |
|---|
| Class | Small Molecule |
|---|
| Description | Isolated from leaves of a blight-resistant rice cultivariety Oryzalic acid B is found in cereals and cereal products. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
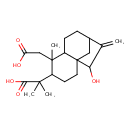
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Oryzalate b | Generator | | 15-Hydroxy-2,3-seco-16-kaurene-2,3-dioic acid | HMDB | | 2-[5-(Carboxymethyl)-11-hydroxy-5-methyl-10-methylidenetricyclo[7.2.1.0¹,⁶]dodecan-4-yl]-2-methylpropanoate | Generator |
|
|---|
| Chemical Formula | C20H30O5 |
|---|
| Average Molecular Mass | 350.449 g/mol |
|---|
| Monoisotopic Mass | 350.209 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2-[5-(carboxymethyl)-11-hydroxy-5-methyl-10-methylidenetricyclo[7.2.1.0¹,⁶]dodecan-4-yl]-2-methylpropanoic acid |
|---|
| Traditional Name | 2-[5-(carboxymethyl)-11-hydroxy-5-methyl-10-methylidenetricyclo[7.2.1.0¹,⁶]dodecan-4-yl]-2-methylpropanoic acid |
|---|
| SMILES | CC(C)(C1CCC23CC(CCC2C1(C)CC(O)=O)C(=C)C3O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C20H30O5/c1-11-12-5-6-14-19(4,10-15(21)22)13(18(2,3)17(24)25)7-8-20(14,9-12)16(11)23/h12-14,16,23H,1,5-10H2,2-4H3,(H,21,22)(H,24,25) |
|---|
| InChI Key | DKUYNSVJKKPQRW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dicarboxylic acids and derivatives. These are organic compounds containing exactly two carboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Dicarboxylic acids and derivatives |
|---|
| Direct Parent | Dicarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dicarboxylic acid or derivatives
- Cyclic alcohol
- Secondary alcohol
- Carboxylic acid
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic homopolycyclic compound
|
|---|
| Molecular Framework | Aliphatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01si-2469000000-7a9b01493e3bfd66fa67 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0fb9-6100940000-f9e683946d41e5480c5a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fsi-0039000000-eaed6eada41b62ee1c5a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05n0-0095000000-8e90750891fb063c2582 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0q2m-1290000000-24fc3d3d655bfefde50e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052b-0029000000-3fd62f49acfbb1959945 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-2079000000-e9de1356025675b6d175 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-9072000000-3af974e27066681d4bc4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-6dc796623f461fdf2a04 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4s-0049000000-40964a404fcce2efe723 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000f-9067000000-d89448f6113e063189fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fsi-0029000000-b81c3659259785042d60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001c-0194000000-7bb770b430f6fb7977b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001l-9460000000-8ef5b5d0b3bb2a0de7d2 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038693 |
|---|
| FooDB ID | FDB018097 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752433 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|