| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:05:09 UTC |
|---|
| Update Date | 2016-11-09 01:19:23 UTC |
|---|
| Accession Number | CHEM032017 |
|---|
| Identification |
|---|
| Common Name | L-3-Amino-2-(oxalylamino)propanoic acid |
|---|
| Class | Small Molecule |
|---|
| Description | L-3-Amino-2-(oxalylamino)propanoic acid is found in pulses. L-3-Amino-2-(oxalylamino)propanoic acid is present in seeds of Lathyrus sativus (chickling pea). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
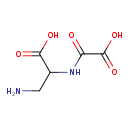
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| L-3-Amino-2-(oxalylamino)propanoate | Generator | | 3-amino-N-(Carboxycarbonyl)-L-alanine | HMDB | | BOAA | HMDB | | Oxalyldiaminopropionic acid | HMDB, MeSH | | 3-oxalylamino-2-Aminopropionic acid | MeSH, HMDB | | Carboxycarbonyl-aminoalanine | MeSH, HMDB | | Lathyrus neurotoxin | MeSH, HMDB | | ODAP | MeSH, HMDB | | beta-N-oxalylamino-L-Alanine | MeSH, HMDB | | beta-N-Oxalylaminoalanine | MeSH, HMDB | | Dencichin | MeSH, HMDB | | Dencichine | MeSH, HMDB | | Oxalyldiaminopropionic acid, (L-ala)-isomer | MeSH, HMDB | | 2-oxalylamino-3-Aminopropionic acid | MeSH, HMDB | | L-BOAA | MeSH, HMDB | | beta-N-Oxalylaminoalanine, (L)-isomer | MeSH, HMDB | | (2-amino-2-Carboxymethyl)-L-oxamic acid | MeSH, HMDB | | Oxalylaminoalanine | MeSH, HMDB | | 3-Amino-2-(carboxyformamido)propanoate | Generator | | 3-Amino-N-(carboxycarbonyl)alanine | MeSH | | 3-Amino-N-(carboxycarbonyl)-DL-alanine | MeSH |
|
|---|
| Chemical Formula | C5H8N2O5 |
|---|
| Average Molecular Mass | 176.127 g/mol |
|---|
| Monoisotopic Mass | 176.043 g/mol |
|---|
| CAS Registry Number | 61277-72-3 |
|---|
| IUPAC Name | 3-amino-2-(carboxyformamido)propanoic acid |
|---|
| Traditional Name | 3-amino-2-(carboxyformamido)propanoic acid |
|---|
| SMILES | NCC(NC(=O)C(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C5H8N2O5/c6-1-2(4(9)10)7-3(8)5(11)12/h2H,1,6H2,(H,7,8)(H,9,10)(H,11,12) |
|---|
| InChI Key | FNXJKVNOUQAQMB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl-alpha amino acids. N-acyl-alpha amino acids are compounds containing an alpha amino acid which bears an acyl group at its terminal nitrogen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | N-acyl-alpha amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-alpha-amino acid
- Dicarboxylic acid or derivatives
- Carboxamide group
- Secondary carboxylic acid amide
- Amino acid
- Carboxylic acid
- Organic oxide
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Primary aliphatic amine
- Organic oxygen compound
- Organic nitrogen compound
- Carbonyl group
- Amine
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001l-9200000000-56fbde8c7c6cc93301f9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0089-9240000000-fde3935c0c77c8255202 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01t9-1900000000-ccc421e99c75411197b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03e9-5900000000-abd0eeaa9ca8a84e4021 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ox-9300000000-71a0d5442e949f525cb4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0900000000-5c969276171b5211bf2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0059-2900000000-bd92dce1bb6935916794 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-5d26e515d958373f8c23 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-0900000000-c8da0ed05a960b36152d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-9300000000-039df959c0d609e980a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-759667e12ce50bf92059 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fc0-2900000000-5efafa93006ef3560a2f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03el-6900000000-05cdb05df7f9f2ba0949 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-2d3df326a2952e51643f | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038668 |
|---|
| FooDB ID | FDB018067 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 97093 |
|---|
| ChEBI ID | 173813 |
|---|
| PubChem Compound ID | 107978 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|