| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:03:50 UTC |
|---|
| Update Date | 2016-11-09 01:19:22 UTC |
|---|
| Accession Number | CHEM031983 |
|---|
| Identification |
|---|
| Common Name | (-)-Dioxibrassinin |
|---|
| Class | Small Molecule |
|---|
| Description | (-)-Dioxibrassinin is found in brassicas. (-)-Dioxibrassinin is an alkaloid from cabbage inoculated with Pseudomonas cichorii. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
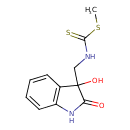
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Dioxibrassinin | HMDB | | N-[(2,3-Dihydroxy-3H-indol-3-yl)methyl](methylsulfanyl)carboimidothioate | Generator | | N-[(2,3-Dihydroxy-3H-indol-3-yl)methyl](methylsulphanyl)carboimidothioate | Generator | | N-[(2,3-Dihydroxy-3H-indol-3-yl)methyl](methylsulphanyl)carboimidothioic acid | Generator |
|
|---|
| Chemical Formula | C11H12N2O2S2 |
|---|
| Average Molecular Mass | 268.355 g/mol |
|---|
| Monoisotopic Mass | 268.034 g/mol |
|---|
| CAS Registry Number | 133761-64-5 |
|---|
| IUPAC Name | N-[(3-hydroxy-2-oxo-2,3-dihydro-1H-indol-3-yl)methyl](methylsulfanyl)carbothioamide |
|---|
| Traditional Name | N-[(3-hydroxy-2-oxo-1H-indol-3-yl)methyl]methylsulfanylcarbothioamide |
|---|
| SMILES | CSC(=S)NCC1(O)C(=O)NC2=C1C=CC=C2 |
|---|
| InChI Identifier | InChI=1S/C11H12N2O2S2/c1-17-10(16)12-6-11(15)7-4-2-3-5-8(7)13-9(11)14/h2-5,15H,6H2,1H3,(H,12,16)(H,13,14) |
|---|
| InChI Key | DXLDPLVGKCAHPY-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta amino acids and derivatives. These are amino acids having a (-NH2) group attached to the beta carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Beta amino acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta amino acid or derivatives
- Indole or derivatives
- Dihydroindole
- Benzenoid
- Tertiary alcohol
- Dithiocarbamic acid ester
- Carboxamide group
- Lactam
- Secondary carboxylic acid amide
- Azacycle
- Organoheterocyclic compound
- Sulfenyl compound
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Organopnictogen compound
- Organic oxygen compound
- Organic oxide
- Carbonyl group
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00xs-4920000000-a5f063a35aef4786117e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-5191000000-f2c3cefa052e8100f94d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-1590000000-e78bec86dcf343acc64c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-1930000000-0ecb55d2ec13fef6d2ab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-07l6-4900000000-25f2be0639ba6b9e147a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-4290000000-42299a002f8efeb41dd2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014m-9780000000-bc1c5f3cf05d8fca6f8a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9300000000-4f3a90c1e6f1fa25f5f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0090000000-fc264598587d2b8ae50c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01di-0790000000-6cb964256e4bbe3b4355 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-009g-7910000000-0fec0c7df407beca0ee2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-bcc6d510502be955bfaf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014j-3970000000-1b9c697cfb245ff8367d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0007-9320000000-d2ce65cb5b35363c3566 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038634 |
|---|
| FooDB ID | FDB018029 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00037061 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 9982363 |
|---|
| ChEBI ID | 173897 |
|---|
| PubChem Compound ID | 11807698 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|