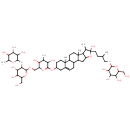| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:02:55 UTC |
|---|
| Update Date | 2016-11-09 01:19:22 UTC |
|---|
| Accession Number | CHEM031964 |
|---|
| Identification |
|---|
| Common Name | Trigofoenoside F |
|---|
| Class | Small Molecule |
|---|
| Description | Trigofoenoside F is found in fenugreek. Trigofoenoside F is isolated from fenugreek seeds. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C51H84O23 |
|---|
| Average Molecular Mass | 1065.199 g/mol |
|---|
| Monoisotopic Mass | 1064.540 g/mol |
|---|
| CAS Registry Number | 94714-56-4 |
|---|
| IUPAC Name | 2-({[4,5-dihydroxy-6-(hydroxymethyl)-3-[(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy]oxan-2-yl]oxy}methyl)-6-{[6-hydroxy-7,9,13-trimethyl-6-(3-methyl-4-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}butyl)-5-oxapentacyclo[10.8.0.0²,⁹.0⁴,⁸.0¹³,¹⁸]icos-18-en-16-yl]oxy}oxane-3,4,5-triol |
|---|
| Traditional Name | 2-({[4,5-dihydroxy-6-(hydroxymethyl)-3-[(3,4,5-trihydroxy-6-methyloxan-2-yl)oxy]oxan-2-yl]oxy}methyl)-6-{[6-hydroxy-7,9,13-trimethyl-6-(3-methyl-4-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}butyl)-5-oxapentacyclo[10.8.0.0²,⁹.0⁴,⁸.0¹³,¹⁸]icos-18-en-16-yl]oxy}oxane-3,4,5-triol |
|---|
| SMILES | CC(CCC1(O)OC2CC3C4CC=C5CC(CCC5(C)C4CCC3(C)C2C1C)OC1OC(COC2OC(CO)C(O)C(O)C2OC2OC(C)C(O)C(O)C2O)C(O)C(O)C1O)COC1OC(CO)C(O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C51H84O23/c1-20(18-66-45-41(62)38(59)34(55)29(16-52)70-45)8-13-51(65)21(2)32-28(74-51)15-27-25-7-6-23-14-24(9-11-49(23,4)26(25)10-12-50(27,32)5)69-47-43(64)39(60)36(57)31(72-47)19-67-48-44(40(61)35(56)30(17-53)71-48)73-46-42(63)37(58)33(54)22(3)68-46/h6,20-22,24-48,52-65H,7-19H2,1-5H3 |
|---|
| InChI Key | BHXVNJJNKMZPQB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as steroidal saponins. These are saponins in which the aglycone moiety is a steroid. The steroidal aglycone is usually a spirostane, furostane, spirosolane, solanidane, or curcubitacin derivative. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Steroidal glycosides |
|---|
| Direct Parent | Steroidal saponins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Steroidal saponin
- Diterpene glycoside
- Furostane-skeleton
- Oligosaccharide
- 22-hydroxysteroid
- Diterpenoid
- Hydroxysteroid
- Delta-5-steroid
- Terpene glycoside
- Fatty acyl glycoside
- Alkyl glycoside
- Glycosyl compound
- O-glycosyl compound
- Fatty acyl
- Oxane
- Tetrahydrofuran
- Secondary alcohol
- Hemiacetal
- Organoheterocyclic compound
- Oxacycle
- Polyol
- Acetal
- Alcohol
- Hydrocarbon derivative
- Organic oxygen compound
- Primary alcohol
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-7301290427-45a3148a0beccd901d57 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f92-1320490404-11cad31ebb655837bc3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002k-5614390203-a1c2700d6b7008ef7d94 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03dj-9611040332-48784f2e198725a270b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dj-3901030011-8efbb4c45c7efa4ee823 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ox-7900180000-0865f60c9df86806203d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-9100000002-ef358948365180da5932 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08fr-9100000012-3e448e06f662ed7bf384 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4m-9100001104-22a70b3c2d50d843d778 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-016s-9000010011-439e9631eab1d9240c77 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01dj-9608280325-0306ae52d7dd8d4bbb1b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4j-9300003116-7a34f36bef85760070e4 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038615 |
|---|
| FooDB ID | FDB018009 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752407 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|