| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:02:53 UTC |
|---|
| Update Date | 2016-11-09 01:19:22 UTC |
|---|
| Accession Number | CHEM031963 |
|---|
| Identification |
|---|
| Common Name | gamma-L-Glutamyl-L-pipecolic acid |
|---|
| Class | Small Molecule |
|---|
| Description | gamma-L-Glutamyl-L-pipecolic acid is found in green vegetables. gamma-L-Glutamyl-L-pipecolic acid is isolated from the seeds of Gleditisia caspica (Caspian locust). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
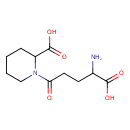
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| g-L-Glutamyl-L-pipecolate | Generator | | g-L-Glutamyl-L-pipecolic acid | Generator | | gamma-L-Glutamyl-L-pipecolate | Generator | | Γ-L-glutamyl-L-pipecolate | Generator | | Γ-L-glutamyl-L-pipecolic acid | Generator | | 1-(4-Amino-4-carboxybutanoyl)piperidine-2-carboxylate | Generator |
|
|---|
| Chemical Formula | C11H18N2O5 |
|---|
| Average Molecular Mass | 258.271 g/mol |
|---|
| Monoisotopic Mass | 258.122 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 1-(4-amino-4-carboxybutanoyl)piperidine-2-carboxylic acid |
|---|
| Traditional Name | 1-(4-amino-4-carboxybutanoyl)piperidine-2-carboxylic acid |
|---|
| SMILES | NC(CCC(=O)N1CCCCC1C(O)=O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C11H18N2O5/c12-7(10(15)16)4-5-9(14)13-6-2-1-3-8(13)11(17)18/h7-8H,1-6,12H2,(H,15,16)(H,17,18) |
|---|
| InChI Key | MNEBDCWSYIPUDZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- Gamma-glutamyl alpha-amino acid
- Glutamine or derivatives
- Alpha-amino acid
- Alpha-amino acid or derivatives
- N-acyl-piperidine
- Piperidinecarboxylic acid
- Heterocyclic fatty acid
- Dicarboxylic acid or derivatives
- Fatty acyl
- Fatty acid
- Piperidine
- Tertiary carboxylic acid amide
- Amino acid or derivatives
- Carboxamide group
- Amino acid
- Carboxylic acid
- Azacycle
- Organoheterocyclic compound
- Primary aliphatic amine
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Amine
- Primary amine
- Organonitrogen compound
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-02bf-9430000000-27e1c9d24fd0ba2e4e35 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00n0-7491000000-2abaa5937e009d2c9b52 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-08fr-0490000000-13b9a4f079afc7e495fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03ea-3950000000-c348f2a015f74af01662 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-053r-9500000000-9b2f1bbe09f76d62a52f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0bt9-0290000000-be81e9ef62a9c1d3f6f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03fr-3980000000-742741e2b5700920d3bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001l-9700000000-1922eeec95820654479e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002r-0890000000-28abcdf27cae21d8bc41 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-2910000000-7e842f1db0df144160bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01t9-5900000000-e89ff87cbcb9d2b62bea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0190000000-eb1361ed45c731b030b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-7930000000-91c7aca764ddf3857ddc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-9200000000-e825f8acf2dd895cd8bc | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038614 |
|---|
| FooDB ID | FDB018008 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35014615 |
|---|
| ChEBI ID | 173838 |
|---|
| PubChem Compound ID | 69247902 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|