| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:02:15 UTC |
|---|
| Update Date | 2016-11-09 01:19:22 UTC |
|---|
| Accession Number | CHEM031950 |
|---|
| Identification |
|---|
| Common Name | Narceinone |
|---|
| Class | Small Molecule |
|---|
| Description | Narceinone is found in opium poppy. Narceinone is an alkaloid from unlanced dried capsules of Papaver somniferum (opium poppy). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
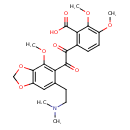
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 6-(2-{6-[2-(dimethylamino)ethyl]-4-methoxy-2H-1,3-benzodioxol-5-yl}-2-oxoacetyl)-2,3-dimethoxybenzoate | HMDB |
|
|---|
| Chemical Formula | C23H25NO9 |
|---|
| Average Molecular Mass | 459.446 g/mol |
|---|
| Monoisotopic Mass | 459.153 g/mol |
|---|
| CAS Registry Number | 125187-48-6 |
|---|
| IUPAC Name | 6-(2-{6-[2-(dimethylamino)ethyl]-4-methoxy-2H-1,3-benzodioxol-5-yl}-2-oxoacetyl)-2,3-dimethoxybenzoic acid |
|---|
| Traditional Name | 6-(2-{6-[2-(dimethylamino)ethyl]-4-methoxy-2H-1,3-benzodioxol-5-yl}-2-oxoacetyl)-2,3-dimethoxybenzoic acid |
|---|
| SMILES | COC1=C(OC)C(C(O)=O)=C(C=C1)C(=O)C(=O)C1=C(OC)C2=C(OCO2)C=C1CCN(C)C |
|---|
| InChI Identifier | InChI=1S/C23H25NO9/c1-24(2)9-8-12-10-15-21(33-11-32-15)22(31-5)16(12)19(26)18(25)13-6-7-14(29-3)20(30-4)17(13)23(27)28/h6-7,10H,8-9,11H2,1-5H3,(H,27,28) |
|---|
| InChI Key | HXFMRYFLZVSZMP-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stilbenes. These are organic compounds containing a 1,2-diphenylethylene moiety. Stilbenes (C6-C2-C6 ) are derived from the common phenylpropene (C6-C3) skeleton building block. The introduction of one or more hydroxyl groups to a phenyl ring lead to stilbenoids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Stilbenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Stilbene
- M-methoxybenzoic acid or derivatives
- O-methoxybenzoic acid or derivatives
- Dimethoxybenzene
- O-dimethoxybenzene
- Benzodioxole
- Benzoic acid or derivatives
- Benzoic acid
- Phenethylamine
- Methoxybenzene
- Aryl ketone
- Anisole
- Phenol ether
- Phenoxy compound
- Benzoyl
- Aralkylamine
- Alkyl aryl ether
- Benzenoid
- Monocyclic benzene moiety
- Alpha-diketone
- Amino acid
- Amino acid or derivatives
- Tertiary aliphatic amine
- Tertiary amine
- Ketone
- Oxacycle
- Organoheterocyclic compound
- Acetal
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Carboxylic acid
- Ether
- Organic oxide
- Amine
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9530500000-1e54bbe78dbb230e38b4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0aor-9230340000-f0d5406a85d117e866f1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0020900000-11db2cbdfc5750a581b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0wml-0163900000-46f3934bc0cc19d9cda1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0umi-0591000000-0381be487e43f4e094cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0bt9-0011900000-fddde9283a2673b33902 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01ot-0328900000-cd7583199ca1741934e3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0005-0219100000-a63e5c30c0d1de07fe11 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-06r5-0004900000-0dd9ea4648c054b066a5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0124900000-8c765f6873e47de32342 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aba-0319600000-b8869b8b91a7cb31570d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000900000-5a39426fb7cfd4e5759a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0007-0054900000-9dbba6aa84f4991ba227 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-6695600000-a7c0c15693e587d7bcb3 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038598 |
|---|
| FooDB ID | FDB017989 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00028646 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30777273 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 14355673 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|