| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:01:06 UTC |
|---|
| Update Date | 2016-11-09 01:19:22 UTC |
|---|
| Accession Number | CHEM031925 |
|---|
| Identification |
|---|
| Common Name | PC-M5' |
|---|
| Class | Small Molecule |
|---|
| Description | Tremorgenic mycotoxin from Penicillium crustosum |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
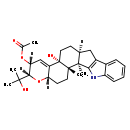
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (1S,2R,5S,7S,8R,11S,14S)-11-Hydroxy-7-(2-hydroxypropan-2-yl)-1,2-dimethyl-6-oxa-23-azahexacyclo[12.10.0.0²,¹¹.0⁵,¹⁰.0¹⁶,²⁴.0¹⁷,²²]tetracosa-9,16(24),17,19,21-pentaen-8-yl acetic acid | Generator |
|
|---|
| Chemical Formula | C29H37NO5 |
|---|
| Average Molecular Mass | 479.608 g/mol |
|---|
| Monoisotopic Mass | 479.267 g/mol |
|---|
| CAS Registry Number | 133613-75-9 |
|---|
| IUPAC Name | (1S,2R,5S,7S,8R,11S,14S)-11-hydroxy-7-(2-hydroxypropan-2-yl)-1,2-dimethyl-6-oxa-23-azahexacyclo[12.10.0.0²,¹¹.0⁵,¹⁰.0¹⁶,²⁴.0¹⁷,²²]tetracosa-9,16(24),17(22),18,20-pentaen-8-yl acetate |
|---|
| Traditional Name | (1S,2R,5S,7S,8R,11S,14S)-11-hydroxy-7-(2-hydroxypropan-2-yl)-1,2-dimethyl-6-oxa-23-azahexacyclo[12.10.0.0²,¹¹.0⁵,¹⁰.0¹⁶,²⁴.0¹⁷,²²]tetracosa-9,16(24),17(22),18,20-pentaen-8-yl acetate |
|---|
| SMILES | [H][C@]12CC3=C(NC4=C3C=CC=C4)[C@]1(C)[C@@]1(C)CC[C@@H]3O[C@@H]([C@H](OC(C)=O)C=C3[C@]1(O)CC2)C(C)(C)O |
|---|
| InChI Identifier | InChI=1S/C29H37NO5/c1-16(31)34-23-15-20-22(35-25(23)26(2,3)32)11-12-27(4)28(5)17(10-13-29(20,27)33)14-19-18-8-6-7-9-21(18)30-24(19)28/h6-9,15,17,22-23,25,30,32-33H,10-14H2,1-5H3/t17-,22-,23+,25-,27+,28+,29+/m0/s1 |
|---|
| InChI Key | IMABPJDBODVJSN-HKZMUHMQSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as naphthopyrans. Naphthopyrans are compounds containing a pyran ring fused to a naphthalene moiety. Furan is a 6 membered-ring non-aromatic ring with five carbon and one oxygen atoms. Naphthalene is a polycyclic aromatic hydrocarbon made up of two fused benzene rings. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Naphthopyrans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Naphthopyrans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Naphthopyran
- Naphthalene
- 3-alkylindole
- Indole
- Indole or derivatives
- Pyran
- Benzenoid
- Cyclic alcohol
- Heteroaromatic compound
- Tertiary alcohol
- Pyrrole
- Carboxylic acid ester
- Carboxylic acid derivative
- Dialkyl ether
- Ether
- Monocarboxylic acid or derivatives
- Oxacycle
- Azacycle
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Alcohol
- Hydrocarbon derivative
- Organic nitrogen compound
- Organonitrogen compound
- Organooxygen compound
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9535700000-0d46baa4883cb11dbaf6 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-053u-3902256000-5570b850923dc49bc11c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-001i-0921200000-4c893ae167eea17cef98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03e9-0000900000-d3509424e590ee1b0d65 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-022c-0001900000-f384c9e5e5a545b067ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01b9-6409500000-4aec3ff38b2ecba489b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-2001900000-df72f310f608064376f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-07y0-4001900000-e944e6fcdcf02ae9a147 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9105200000-6aee54df787848d58290 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-9000400000-69e391b682a4bd24e5ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9000000000-c01bbbf5bed889264ddb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9001200000-8eafcf07dd78ec7bab3a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0000900000-d8880341143a327398a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-1505900000-8074fe24ab37b426e54a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kus-2947100000-62944947219cd1abb4d9 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038569 |
|---|
| FooDB ID | FDB017956 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 22943202 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 38348556 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|