| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 03:00:51 UTC |
|---|
| Update Date | 2016-11-09 01:19:22 UTC |
|---|
| Accession Number | CHEM031918 |
|---|
| Identification |
|---|
| Common Name | 8-Propanoylneosolaniol |
|---|
| Class | Small Molecule |
|---|
| Description | 8-Propanoylneosolaniol is produced by Fusarium sporotrichioides. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
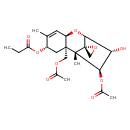
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-(3-Hydroxy-4-methoxybenzyl)-6,7-dimethoxyisoquinoline | HMDB | | 3'-Hydroxypapaverine | HMDB | | (1's,2S,2'r,4's,7'r,9'r,10'r,11's)-11'-(Acetyloxy)-2'-[(acetyloxy)methyl]-10'-hydroxy-1',5'-dimethyl-8'-oxaspiro[oxirane-2,12'-tricyclo[7.2.1.0²,⁷]dodecan]-5'-en-4'-yl propanoic acid | HMDB |
|
|---|
| Chemical Formula | C22H30O9 |
|---|
| Average Molecular Mass | 438.468 g/mol |
|---|
| Monoisotopic Mass | 438.189 g/mol |
|---|
| CAS Registry Number | 111112-47-1 |
|---|
| IUPAC Name | (1'S,2S,2'R,4'S,7'R,9'R,10'R,11'S)-11'-(acetyloxy)-2'-[(acetyloxy)methyl]-10'-hydroxy-1',5'-dimethyl-8'-oxaspiro[oxirane-2,12'-tricyclo[7.2.1.0²,⁷]dodecan]-5'-en-4'-yl propanoate |
|---|
| Traditional Name | (1'S,2S,2'R,4'S,7'R,9'R,10'R,11'S)-11'-(acetyloxy)-2'-[(acetyloxy)methyl]-10'-hydroxy-1',5'-dimethyl-8'-oxaspiro[oxirane-2,12'-tricyclo[7.2.1.0²,⁷]dodecan]-5'-en-4'-yl propanoate |
|---|
| SMILES | CCC(=O)O[C@H]1C[C@@]2(COC(C)=O)[C@H](O[C@@H]3[C@H](O)[C@@H](OC(C)=O)[C@@]2(C)[C@]32CO2)C=C1C |
|---|
| InChI Identifier | InChI=1S/C22H30O9/c1-6-16(25)30-14-8-21(9-27-12(3)23)15(7-11(14)2)31-19-17(26)18(29-13(4)24)20(21,5)22(19)10-28-22/h7,14-15,17-19,26H,6,8-10H2,1-5H3/t14-,15+,17+,18+,19+,20+,21+,22-/m0/s1 |
|---|
| InChI Key | ZSGWJLBSFZRJAT-MLXHEQMXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as trichothecenes. These are sesquiterpene mycotoxins structurally characterized by the presence of an epoxide ring and a benzopyran derivative with a variant number of hydroxyl, acetyl, or other substituents. The most important structural features causing the biological activities of trichothecenes are the 12,13-epoxy ring, the presence of hydroxyl or acetyl groups at appropriate positions on the trichothecene nucleus and the structure and position of the side-chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Trichothecenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Trichothecene skeleton
- Tricarboxylic acid or derivatives
- Oxepane
- Oxane
- Cyclic alcohol
- Secondary alcohol
- Carboxylic acid ester
- Oxacycle
- Ether
- Oxirane
- Dialkyl ether
- Carboxylic acid derivative
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Organooxygen compound
- Alcohol
- Carbonyl group
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0403-4049000000-d115b39630aeece1635e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-000i-8059800000-54f93b86e5df08994ae5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05ds-4009400000-cb9858b3587b7109f82c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ab9-4209100000-00b78b6544f99f24dfbc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ab9-9108000000-30c90badecf716957733 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000j-5009600000-6be102a2992ef60f2bf8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-8209200000-bb8761e416df51d65d44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4j-6900000000-0dd057b43862d711226e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0ab9-0009000000-45d686b7a96ff33c2190 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1009000000-ef8eb403238bd216a269 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05mx-9012000000-646eaa3cabdea128de0b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-6019200000-9d24468b96c21b6d10bd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0abi-9003000000-b423fec64f40bb621b97 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-008c-9033000000-0220a8fed4c232238bee | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038562 |
|---|
| FooDB ID | FDB017949 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00056850 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 159277 |
|---|
| ChEBI ID | 180728 |
|---|
| PubChem Compound ID | 167736 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|