| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:58:56 UTC |
|---|
| Update Date | 2016-11-09 01:19:21 UTC |
|---|
| Accession Number | CHEM031872 |
|---|
| Identification |
|---|
| Common Name | Dinophysistoxin 2 |
|---|
| Class | Small Molecule |
|---|
| Description | Dinophysistoxin 2 is found in mollusks. Dinophysistoxin 2 is a metabolite of Dinophysis species. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
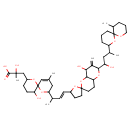
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Dinophysistoxin-2 | HMDB | | DTX-2 | HMDB | | DTX2 | HMDB | | 2-Hydroxy-3-{5-hydroxy-8-[(3E)-4-[8'-hydroxy-6'-(1-hydroxy-3-{11-methyl-1,7-dioxaspiro[5.5]undecan-2-yl}butyl)-7'-methylidene-hexahydro-3'H-spiro[oxolane-2,2'-pyrano[3,2-b]pyran]-5-yl]but-3-en-2-yl]-10-methyl-1,7-dioxaspiro[5.5]undec-10-en-2-yl}-2-methylpropanoate | Generator | | Dinophysistoxin 2 | MeSH |
|
|---|
| Chemical Formula | C44H68O13 |
|---|
| Average Molecular Mass | 805.003 g/mol |
|---|
| Monoisotopic Mass | 804.466 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2-hydroxy-3-{5-hydroxy-8-[(3E)-4-[8'-hydroxy-6'-(1-hydroxy-3-{11-methyl-1,7-dioxaspiro[5.5]undecan-2-yl}butyl)-7'-methylidene-hexahydro-3'H-spiro[oxolane-2,2'-pyrano[3,2-b]pyran]-5-yl]but-3-en-2-yl]-10-methyl-1,7-dioxaspiro[5.5]undec-10-en-2-yl}-2-methylpropanoic acid |
|---|
| Traditional Name | 2-hydroxy-3-{5-hydroxy-8-[(3E)-4-[8'-hydroxy-6'-(1-hydroxy-3-{11-methyl-1,7-dioxaspiro[5.5]undecan-2-yl}butyl)-7'-methylidene-hexahydrospiro[oxolane-2,2'-pyrano[3,2-b]pyran]-5-yl]but-3-en-2-yl]-10-methyl-1,7-dioxaspiro[5.5]undec-10-en-2-yl}-2-methylpropanoic acid |
|---|
| SMILES | CC(CC(O)C1OC2CCC3(CCC(O3)\C=C\C(C)C3CC(C)=CC4(OC(CC(C)(O)C(O)=O)CCC4O)O3)OC2C(O)C1=C)C1CCCC2(OCCCC2C)O1 |
|---|
| InChI Identifier | InChI=1S/C44H68O13/c1-25-21-35(56-44(23-25)36(46)14-13-31(54-44)24-41(6,50)40(48)49)26(2)11-12-30-15-18-42(53-30)19-16-34-39(57-42)37(47)29(5)38(52-34)32(45)22-27(3)33-10-7-17-43(55-33)28(4)9-8-20-51-43/h11-12,23,26-28,30-39,45-47,50H,5,7-10,13-22,24H2,1-4,6H3,(H,48,49)/b12-11+ |
|---|
| InChI Key | BRFKTXCAUCYQBT-VAWYXSNFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as ketals. These are acetals derived from ketones by replacement of the oxo group by two hydrocarbyloxy groups R2C(OR)2 ( R not Hydrogen ). This term, once abandoned, has been reinstated as a subclass of acetals. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Ethers |
|---|
| Direct Parent | Ketals |
|---|
| Alternative Parents | |
|---|
| Substituents | - Ketal
- Alpha-hydroxy acid
- Hydroxy acid
- Oxane
- Pyran
- Tertiary alcohol
- Tetrahydrofuran
- Secondary alcohol
- Organoheterocyclic compound
- Oxacycle
- Monocarboxylic acid or derivatives
- Dialkyl ether
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Alcohol
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-5302016920-21ce3dc22deba7854c97 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ku-7133286900-bf1f07ad7baf40185656 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f6x-9311145100-c32875ac3a3b88372df8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-9302111110-09d9fd7c4aae10e15506 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-9218000600-ef0ab3d8496f21340834 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0jdi-3935110100-2a7912d3ad329b0eb0b2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0000000390-9b7835256454ae9753f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-2000002790-6a88f6fa1a72731f9865 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0umj-9243705650-2a577549974c7360cea0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0000002980-51fe23d20a098119810d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0132315940-54eefbb4dd1e509894ef | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00kk-8900005110-e4e5b1a4f8802759de14 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038517 |
|---|
| FooDB ID | FDB017897 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4941670 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6437082 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|