| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:58:41 UTC |
|---|
| Update Date | 2016-11-09 01:19:21 UTC |
|---|
| Accession Number | CHEM031864 |
|---|
| Identification |
|---|
| Common Name | Rheidin B |
|---|
| Class | Small Molecule |
|---|
| Description | Rheidin B is found in green vegetables. Rheidin B is isolated from rhubarb roots. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
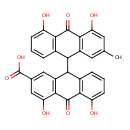
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Reidin b | HMDB | | 4,4',5,5'-Tetrahydroxy-2'-methyl-10,10'-dioxo-9H,9'H,10H,10'H-[9,9'-bianthracene]-2-carboxylate | Generator |
|
|---|
| Chemical Formula | C30H20O8 |
|---|
| Average Molecular Mass | 508.475 g/mol |
|---|
| Monoisotopic Mass | 508.116 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 9-(4,5-dihydroxy-2-methyl-10-oxo-9,10-dihydroanthracen-9-yl)-4,5-dihydroxy-10-oxo-9,10-dihydroanthracene-2-carboxylic acid |
|---|
| Traditional Name | 9-(4,5-dihydroxy-2-methyl-10-oxo-9H-anthracen-9-yl)-4,5-dihydroxy-10-oxo-9H-anthracene-2-carboxylic acid |
|---|
| SMILES | CC1=CC2=C(C(O)=C1)C(=O)C1=C(C=CC=C1O)C2C1C2=C(C(O)=CC=C2)C(=O)C2=C1C=C(C=C2O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C30H20O8/c1-12-8-16-22(14-4-2-6-18(31)24(14)28(35)26(16)20(33)9-12)23-15-5-3-7-19(32)25(15)29(36)27-17(23)10-13(30(37)38)11-21(27)34/h2-11,22-23,31-34H,1H3,(H,37,38) |
|---|
| InChI Key | BYPYDHLERFKKKV-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as anthracenecarboxylic acids. These are organic compounds containing a carboxylic acid group attached to an anthracene ring system. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Anthracenes |
|---|
| Sub Class | Anthracenecarboxylic acids and derivatives |
|---|
| Direct Parent | Anthracenecarboxylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Anthracene carboxylic acid
- 2-naphthalenecarboxylic acid
- 2-naphthalenecarboxylic acid or derivatives
- Hydroxybenzoic acid
- Aryl ketone
- 1-hydroxy-2-unsubstituted benzenoid
- 1-hydroxy-4-unsubstituted benzenoid
- Vinylogous acid
- Ketone
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxygen compound
- Organic oxide
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0043-0130900000-9d64f30f3688c5a16fea | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-007c-2112059000-9c2f3e6aec14a01b8e50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4l-0000960000-3ff6ec191accfb547d5f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01vp-0221910000-39232e8d2b9d2f48dba2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-006t-0433900000-198f78e02c58aebc7695 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000590000-ba6960427bd5073ae27a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-08fr-0000940000-46834ec1901c33559ef1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01pd-1004900000-c95a23a20ba6646712f1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4l-0000590000-9c333959c53c6f832650 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4l-0000590000-eb8181d95dad160a4368 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01ql-1012900000-ac07234c6d499e2ce119 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0000290000-36cbfee3120a657fb13c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0000490000-3bfd10a7ec99d6634ab9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-0000910000-622f7de545bb5679b154 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038509 |
|---|
| FooDB ID | FDB017886 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4478878 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5320958 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|