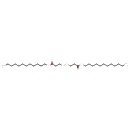| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:56:59 UTC |
|---|
| Update Date | 2016-11-09 01:19:20 UTC |
|---|
| Accession Number | CHEM031826 |
|---|
| Identification |
|---|
| Common Name | Dilauryl 3,3'-thiodipropionate |
|---|
| Class | Small Molecule |
|---|
| Description | Dilauryl 3,3'-thiodipropionate is a food and polymer antioxidant. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Dilauryl 3,3'-thiodipropionic acid | Generator | | Dilauryl 3,3'-dithiodipropionate | HMDB | | Propanoic acid, 3,3'-dithiobis-, 1,1'-didodecyl ester | HMDB | | Propanoic acid, 3,3'-dithiobis-, didodecyl ester (9ci) | HMDB | | Propionic acid, 3,3'-dithiodi-, didodecyl ester (7ci,8ci) | HMDB | | Dodecyl 3-{[3-(dodecyloxy)-3-oxopropyl]disulfanyl}propanoic acid | Generator | | Dodecyl 3-{[3-(dodecyloxy)-3-oxopropyl]disulphanyl}propanoate | Generator | | Dodecyl 3-{[3-(dodecyloxy)-3-oxopropyl]disulphanyl}propanoic acid | Generator |
|
|---|
| Chemical Formula | C30H58O4S2 |
|---|
| Average Molecular Mass | 546.909 g/mol |
|---|
| Monoisotopic Mass | 546.378 g/mol |
|---|
| CAS Registry Number | 5926-55-6 |
|---|
| IUPAC Name | dodecyl 3-{[3-(dodecyloxy)-3-oxopropyl]disulfanyl}propanoate |
|---|
| Traditional Name | dodecyl 3-{[3-(dodecyloxy)-3-oxopropyl]disulfanyl}propanoate |
|---|
| SMILES | CCCCCCCCCCCCOC(=O)CCSSCCC(=O)OCCCCCCCCCCCC |
|---|
| InChI Identifier | InChI=1S/C30H58O4S2/c1-3-5-7-9-11-13-15-17-19-21-25-33-29(31)23-27-35-36-28-24-30(32)34-26-22-20-18-16-14-12-10-8-6-4-2/h3-28H2,1-2H3 |
|---|
| InChI Key | OHCIBJNNZNPROG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dicarboxylic acids and derivatives. These are organic compounds containing exactly two carboxylic acid groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Dicarboxylic acids and derivatives |
|---|
| Direct Parent | Dicarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dicarboxylic acid or derivatives
- Dialkyldisulfide
- Organic disulfide
- Carboxylic acid ester
- Sulfenyl compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-02tc-5936000000-8f4b2e9ac57684755d9e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0544090000-ea2f353d8dfdece3c196 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-2922000000-77df86e8a1114cee4f8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-5910200000-6a352928072f2da6293f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-052b-0429060000-39dca6f36e184ae8bb88 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dr-5894010000-6fff342cf1bfc9367d10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0aou-1923000000-f41bdbe013c6bf004428 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-3012090000-e17a8d1f5bd2f658024f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-054k-9616460000-aec836c934f9fd214137 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9300000000-8d0a96673fbb012d0f69 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0145090000-173826ef6c5c892bc0be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kmi-6795030000-39b1953746793175ba26 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-0429100000-2dbd9fb59a4dceee7089 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038474 |
|---|
| FooDB ID | FDB017838 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30777257 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 24758198 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|