| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:52:53 UTC |
|---|
| Update Date | 2016-11-09 01:19:19 UTC |
|---|
| Accession Number | CHEM031732 |
|---|
| Identification |
|---|
| Common Name | Neoglucobrassicin |
|---|
| Class | Small Molecule |
|---|
| Description | Widespread in Brassica subspecies and found in the Cruciferae, Tovariaceae, Capparidaceae and Resedaceae |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
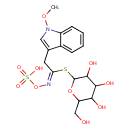
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1-Methoxy-3-indolylmethyl glucosinolate | HMDB | | 1-Methoxy-3-indolylmethylglucosinolate | HMDB | | 1-Methoxyindol-3-ylmethylglucosinolate | HMDB | | MIMG | HMDB | | N-Methoxyglucobrassicin | HMDB | | NGBS CPD | MeSH |
|
|---|
| Chemical Formula | C17H22N2O10S2 |
|---|
| Average Molecular Mass | 478.494 g/mol |
|---|
| Monoisotopic Mass | 478.072 g/mol |
|---|
| CAS Registry Number | 5187-84-8 |
|---|
| IUPAC Name | {[(E)-[2-(1-methoxy-1H-indol-3-yl)-1-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulfanyl}ethylidene]amino]oxy}sulfonic acid |
|---|
| Traditional Name | [(E)-[2-(1-methoxyindol-3-yl)-1-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]sulfanyl}ethylidene]amino]oxysulfonic acid |
|---|
| SMILES | CON1C=C(C\C(SC2OC(CO)C(O)C(O)C2O)=N/OS(O)(=O)=O)C2=CC=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C17H22N2O10S2/c1-27-19-7-9(10-4-2-3-5-11(10)19)6-13(18-29-31(24,25)26)30-17-16(23)15(22)14(21)12(8-20)28-17/h2-5,7,12,14-17,20-23H,6,8H2,1H3,(H,24,25,26)/b18-13+ |
|---|
| InChI Key | PKKMITFKYRCCOL-QGOAFFKASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alkylglucosinolates. These are organic compounds containing a glucosinolate moiety that carries an alkyl chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Alkylglucosinolates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkylglucosinolate
- Glycosyl compound
- S-glycosyl compound
- 3-alkylindole
- Indole
- Indole or derivatives
- Oxane
- Benzenoid
- Substituted pyrrole
- Monothioacetal
- Heteroaromatic compound
- Organic sulfuric acid or derivatives
- Pyrrole
- Secondary alcohol
- Oxacycle
- Azacycle
- Polyol
- Organoheterocyclic compound
- Sulfenyl compound
- Organosulfur compound
- Organonitrogen compound
- Alcohol
- Hydrocarbon derivative
- Organic nitrogen compound
- Primary alcohol
- Organopnictogen compound
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0c01-9301500000-d2247ce6c0e0546630d5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00o0-3263119000-a47e64f5b0b0fc458e80 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-02dj-0605900000-c3cdf47795a38d1752cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-030s-0549000000-1b7b930d214a1b08f22c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0bt9-9311000000-25ff4f0aee49e7953596 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-3239100000-35cbf9d3d94b358848b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ue9-4290000000-122cd0956ee108348b92 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-8970000000-6a9a0cad3f007145f875 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038384 |
|---|
| FooDB ID | FDB017734 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00000126 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 27506 |
|---|
| PubChem Compound ID | 6828856 |
|---|
| Kegg Compound ID | C08424 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|