| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:52:33 UTC |
|---|
| Update Date | 2016-11-09 01:19:19 UTC |
|---|
| Accession Number | CHEM031726 |
|---|
| Identification |
|---|
| Common Name | Triton X 100 |
|---|
| Class | Small Molecule |
|---|
| Description | Triton X 100 is listed in the EAFUS Food Additive Database (Jan. 2001) but with no reported uses.Triton X-100 (C14H22O(C2H4O)n) is a nonionic surfactant which has a hydrophilic polyethylene oxide group (on average it has 9.5 ethylene oxide units) and a hydrocarbon lipophilic or hydrophobic group. The hydrocarbon group is a 4-(1,1,3,3-tetramethylbutyl)-phenyl group. (Wikipedia). |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
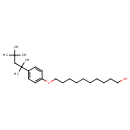
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-((1,1,3,3-Tetramethylbutyl)phenoxy)ethanol | HMDB | | 2-(4-(1,1,3,3-Tetramethylbutyl)phenoxy)ethanol | HMDB | | 2-(P-(1,1,3,3-Tetramethylbutyl)phenoxy)ethanol | HMDB | | 4-(1,1,3,3-Tetramethylbutyl)phenol, ethoxylated | HMDB | | 4-(1,1,3,3-Tetramethylbutyl)phenyl hydroxypoly(oxyethylene) | HMDB | | 4-Tert-octylphenyl peg ether | HMDB | | Antarox a-200 | HMDB | | Ethoxylated P-tert-octylphenol | HMDB | | Hydrol SW | HMDB | | Octoxynol 5 | HMDB | | Octylphenoxy-ethanol | HMDB | | Octylphenoxypolyethoxyethanol | HMDB | | Polyethylene glycol mono(4-octylphenyl) ether | HMDB | | Polyethylene glycol mono(4-tert-octylphenyl) ether | HMDB | | Polyethylene glycol mono(octylphenyl) ether | HMDB | | Polyethylene glycol mono(P-tert-octylphenyl) ether | HMDB | | Polyethylene glycol monoether with P-tert-octylphenyl | HMDB | | Polyethylene glycol octylphenol ether | HMDB | | Polyethylene glycol P-1,1,3,3-tetramethylbutylphenyl ether | HMDB | | Polyethylene glycol P-tert-octylphenyl ether | HMDB | | Polyethylene glycol tert-octylphenyl ether | HMDB | | Polyethyleneglycol 4-(tert-octyl)phenyl ether | HMDB | | Polyoxyethylene 4-(1,1,3,3-tetramethylbutyl)phenyl ether | HMDB | | Polyoxyethylene octyl phenyl ether | HMDB | | Polyoxysthylene mono(octylphenyl) ether | HMDB | | Preceptin | HMDB | | Texofor FP 300 | HMDB |
|
|---|
| Chemical Formula | C24H42O2 |
|---|
| Average Molecular Mass | 362.589 g/mol |
|---|
| Monoisotopic Mass | 362.318 g/mol |
|---|
| CAS Registry Number | 9002-93-1 |
|---|
| IUPAC Name | 10-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]decan-1-ol |
|---|
| Traditional Name | 10-[4-(2,4,4-trimethylpentan-2-yl)phenoxy]decan-1-ol |
|---|
| SMILES | CC(C)(C)CC(C)(C)C1=CC=C(OCCCCCCCCCCO)C=C1 |
|---|
| InChI Identifier | InChI=1S/C24H42O2/c1-23(2,3)20-24(4,5)21-14-16-22(17-15-21)26-19-13-11-9-7-6-8-10-12-18-25/h14-17,25H,6-13,18-20H2,1-5H3 |
|---|
| InChI Key | YHBHQYRDAVETGQ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as fatty alcohols. These are aliphatic alcohols consisting of a chain of a least six carbon atoms. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Fatty alcohols |
|---|
| Direct Parent | Fatty alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylpropane
- Fatty alcohol
- Phenoxy compound
- Phenol ether
- Alkyl aryl ether
- Benzenoid
- Monocyclic benzene moiety
- Ether
- Organic oxygen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052f-7975000000-518697415211b9cfbe61 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00y0-8954500000-b56aef0e81cf05ca791b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dj-0219000000-7b5d31de8f34ad114bf5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0btj-3789000000-85e6ec92d79de80e2903 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0bt9-7920000000-0852181b7220d78408be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0039000000-a41b4a4605171d74f61b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0196000000-c32f48ee3bae9bbf4516 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-1950000000-df316ded26e3e05735de | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01p9-1196000000-89fd70ce3f620fab5b56 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a5j-7492000000-15de63693bfb34ad641d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-3900000000-035e7a652b34f29ccffe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0009000000-a1ff3fc993337d4da646 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0bt9-0196000000-421fe9b14d87750e6258 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-0900000000-2be3da1d53c9b789ea3e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038380 |
|---|
| FooDB ID | FDB017723 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 30777247 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131752352 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|