| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:51:45 UTC |
|---|
| Update Date | 2016-11-09 01:19:19 UTC |
|---|
| Accession Number | CHEM031710 |
|---|
| Identification |
|---|
| Common Name | Gallocatechin |
|---|
| Class | Small Molecule |
|---|
| Description | A gallocatechin that has (2R,3S)-configuration. It is found in green tea and bananas. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
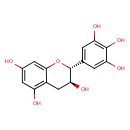
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-Gallocatechol | ChEBI | | (2R,3S)-(+)-Gallocatechin | ChEBI | | (2R,3S)-Flavan-3,3',4',5,5',7-hexol | ChEBI | | (2R,3S)-Flavan-3,5,7,3',4',5'-hexol | ChEBI | | (2R,3S)-Gallocatechin | ChEBI | | Gallocatechin | ChEBI | | Gallocatechol | Kegg | | Epigallocatechin | MeSH | | Epigallocatechol | MeSH | | Gallocatechol, (2R-cis)-isomer | MeSH | | Gallocatechol, (2R-trans)-isomer | MeSH | | Gallocatechol, (2S-trans)-isomer | MeSH | | (+)-trans-3,3',4',5,5',7-Hexahydroxyflavan | HMDB | | Casuarin | HMDB | | d-Gallocatechin | HMDB | | (2R,3S)-3,4-Dihydro-2-(3,4,5-trihydroxyphenyl)-2H-1-benzopyran-3,5,7-triol | PhytoBank | | (+)-Gallocatechin | PhytoBank | | NSC 674038 | PhytoBank | | d-Gallocatechol | PhytoBank |
|
|---|
| Chemical Formula | C15H14O7 |
|---|
| Average Molecular Mass | 306.270 g/mol |
|---|
| Monoisotopic Mass | 306.074 g/mol |
|---|
| CAS Registry Number | 970-73-0 |
|---|
| IUPAC Name | (2R,3S)-2-(3,4,5-trihydroxyphenyl)-3,4-dihydro-2H-1-benzopyran-3,5,7-triol |
|---|
| Traditional Name | (+)-gallocatechin |
|---|
| SMILES | O[C@H]1CC2=C(O)C=C(O)C=C2O[C@@H]1C1=CC(O)=C(O)C(O)=C1 |
|---|
| InChI Identifier | InChI=1S/C15H14O7/c16-7-3-9(17)8-5-12(20)15(22-13(8)4-7)6-1-10(18)14(21)11(19)2-6/h1-4,12,15-21H,5H2/t12-,15+/m0/s1 |
|---|
| InChI Key | XMOCLSLCDHWDHP-SWLSCSKDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as epigallocatechins. Epigallocatechins are compounds containing epigallocatechin or a derivative. Epigallocatechin is a flavan-3-ol containing a benzopyran-3,5,7-triol linked to a 3,4,5-hydroxyphenyl moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Flavans |
|---|
| Direct Parent | Epigallocatechins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Epigallocatechin
- 3'-hydroxyflavonoid
- 3-hydroxyflavonoid
- 4'-hydroxyflavonoid
- 5-hydroxyflavonoid
- 7-hydroxyflavonoid
- Hydroxyflavonoid
- 1-benzopyran
- Chromane
- Benzopyran
- Pyrogallol derivative
- Benzenetriol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Phenol
- Benzenoid
- Monocyclic benzene moiety
- Secondary alcohol
- Polyol
- Organoheterocyclic compound
- Oxacycle
- Ether
- Hydrocarbon derivative
- Alcohol
- Organic oxygen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-0940000000-7217377fccff4c30649e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-0udi-3110049000-8b1040defcae8063c769 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-TOF , negative | splash10-002r-0900000000-c1cd10a5867d444bd1d3 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-TOF , negative | splash10-00n0-0910000000-06ff48cb1edb79736ede | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-TOF , negative | splash10-0a4i-0019000000-83d961b9674b7b1b2a65 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-TOF , negative | splash10-00p0-0941000000-47f58a4dd33c3125d051 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0729000000-fffbd456b9b51c7d0274 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0911000000-203a6a86214265bc09e4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00ds-2900000000-023b154fc2d731ecee10 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0219000000-db1826421845ecab1485 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05n0-0922000000-86fb5a0b2fc660815604 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056r-2910000000-2375ce05cd5da757763c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0019000000-b20f152234d6d77dcaa2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0894000000-05dea610b2f972a7651e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-1930000000-7c643c8857e1259ab4fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0029000000-3578b95ca9c1d3f41df2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05n0-0902000000-291e12c32e9cfe57384b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-1920000000-c1afce079042673ae394 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038365 |
|---|
| FooDB ID | FDB017705 |
|---|
| Phenol Explorer ID | 126 |
|---|
| KNApSAcK ID | C00008817 C00035626 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 58594 |
|---|
| ChEBI ID | 31018 |
|---|
| PubChem Compound ID | 65084 |
|---|
| Kegg Compound ID | C12127 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|