| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:46:50 UTC |
|---|
| Update Date | 2016-11-09 01:19:18 UTC |
|---|
| Accession Number | CHEM031612 |
|---|
| Identification |
|---|
| Common Name | Azaspiracid 2 |
|---|
| Class | Small Molecule |
|---|
| Description | Stress metabolite of Ipomoea batatas (sweet potato). (2E,6E)-1-Hydroxy-2,6,10-farnesatrien-9-one is found in root vegetables and potato. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
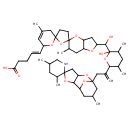
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (4E)-5-(2-{hydroxy[2-hydroxy-3,5-dimethyl-6-(3-{3,5,10'-trimethyl-3',7',12'-trioxaspiro[piperidine-2,4'-tricyclo[6.3.1.0²,⁶]dodecane]-8'-yl}prop-1-en-2-yl)oxan-2-yl]methyl}-4'',6-dimethyl-2,3,3'',3a,6,6'',7,7a-octahydrodispiro[furo[3,2-b]pyran-5,2'-oxolane-5',2''-pyran]-6''-yl)pent-4-enoate | Generator | | Azaspiracid-2 | MeSH |
|
|---|
| Chemical Formula | C48H73NO12 |
|---|
| Average Molecular Mass | 856.093 g/mol |
|---|
| Monoisotopic Mass | 855.513 g/mol |
|---|
| CAS Registry Number | 265996-92-7 |
|---|
| IUPAC Name | (4E)-5-(2-{hydroxy[2-hydroxy-3,5-dimethyl-6-(3-{3,5,10'-trimethyl-3',7',12'-trioxaspiro[piperidine-2,4'-tricyclo[6.3.1.0²,⁶]dodecane]-8'-yl}prop-1-en-2-yl)oxan-2-yl]methyl}-4'',6-dimethyl-2,3,3'',3a,6,6'',7,7a-octahydrodispiro[furo[3,2-b]pyran-5,2'-oxolane-5',2''-pyran]-6''-yl)pent-4-enoic acid |
|---|
| Traditional Name | (4E)-5-(2-{hydroxy[2-hydroxy-3,5-dimethyl-6-(3-{3,5,10'-trimethyl-3',7',12'-trioxaspiro[piperidine-2,4'-tricyclo[6.3.1.0²,⁶]dodecane]-8'-yl}prop-1-en-2-yl)oxan-2-yl]methyl}-4'',6-dimethyl-2,3,3'',3a,6,6'',7,7a-octahydrodispiro[furo[3,2-b]pyran-5,2'-oxolane-5',2''-pyran]-6''-yl)pent-4-enoic acid |
|---|
| SMILES | CC1CNC2(CC3OC4(CC(=C)C5OC(O)(C(O)C6CC7OC8(CCC9(CC(C)=CC(O9)\C=C\CCC(O)=O)O8)C(C)CC7O6)C(C)CC5C)CC(C)CC(O4)C3O2)C(C)C1 |
|---|
| InChI Identifier | InChI=1S/C48H73NO12/c1-26-16-34(11-9-10-12-40(50)51)55-44(21-26)13-14-47(61-44)32(7)19-35-36(58-47)20-38(54-35)43(52)48(53)33(8)18-29(4)41(60-48)30(5)23-45-22-27(2)17-37(56-45)42-39(57-45)24-46(59-42)31(6)15-28(3)25-49-46/h9,11,16,27-29,31-39,41-43,49,52-53H,5,10,12-15,17-25H2,1-4,6-8H3,(H,50,51)/b11-9+ |
|---|
| InChI Key | FWMJPUBOGPIFOU-PKNBQFBNSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sesquiterpenoids. These are terpenes with three consecutive isoprene units. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Sesquiterpenoids |
|---|
| Direct Parent | Sesquiterpenoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sesquiterpenoid
- Farsesane sesquiterpenoid
- Fatty alcohol
- Fatty acyl
- Acryloyl-group
- Enone
- Alpha,beta-unsaturated ketone
- Ketone
- Primary alcohol
- Organooxygen compound
- Organic oxygen compound
- Alcohol
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0khi-0509410040-6e370224026ff6849dfc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0089-1709021100-b0a5ee6b26286eda7bb7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0100-3901000000-f32a2113b551b6abe4e5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0gxt-2831981040-022e1459577d65f54fdb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00bl-0384631290-f873a04c4411035b047b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-1910000000-99ec8345fb81011a8a67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0001001090-c2a279d718daef2d841f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zfr-0007610090-ca90305caa2f4380d49a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fk9-0901200530-5a909cbbd855cce65bc3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0000010090-f3eb54f44f8c6c76244c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-3032200090-4d641c0ec89b6066d495 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05ir-2490002010-069e761ee6463e09d476 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0030891 |
|---|
| FooDB ID | FDB002855 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 101648402 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|