| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-26 02:44:47 UTC |
|---|
| Update Date | 2016-11-09 01:19:17 UTC |
|---|
| Accession Number | CHEM031564 |
|---|
| Identification |
|---|
| Common Name | Peperinic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Peperinic acid is found in herbs and spices. Peperinic acid is isolated from aged peppermint oil. |
|---|
| Contaminant Sources | |
|---|
| Contaminant Type | Not Available |
|---|
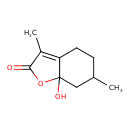
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Peperinate | Generator | | (-)-Menthofuran oxide | HMDB | | 2,4,5,6,7,7a-hexahydro-7a-Hydroxy-3,6-dimethyl-2-benzofuranone | HMDB | | 3-Hydroxy-4(8)-ene-P-menthane-3(9)-lactone | HMDB | | 3-Hydroxy-P-menth-4(8)-en-9,3-olide | HMDB | | D-Menthofuranoxide | HMDB |
|
|---|
| Chemical Formula | C10H14O3 |
|---|
| Average Molecular Mass | 182.216 g/mol |
|---|
| Monoisotopic Mass | 182.094 g/mol |
|---|
| CAS Registry Number | 514-93-2 |
|---|
| IUPAC Name | 7a-hydroxy-3,6-dimethyl-2,4,5,6,7,7a-hexahydro-1-benzofuran-2-one |
|---|
| Traditional Name | 7a-hydroxy-3,6-dimethyl-4,5,6,7-tetrahydro-1-benzofuran-2-one |
|---|
| SMILES | CC1CCC2=C(C)C(=O)OC2(O)C1 |
|---|
| InChI Identifier | InChI=1S/C10H14O3/c1-6-3-4-8-7(2)9(11)13-10(8,12)5-6/h6,12H,3-5H2,1-2H3 |
|---|
| InChI Key | LBNWZGLSMCTAQB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzofurans. These are organic compounds containing a benzene ring fused to a furan. Furan is a five-membered aromatic ring with four carbon atoms and one oxygen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzofurans |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzofurans |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzofuran
- 2-furanone
- Cyclic alcohol
- Dihydrofuran
- Alpha,beta-unsaturated carboxylic ester
- Enoate ester
- Carboxylic acid ester
- Hemiacetal
- Lactone
- Oxacycle
- Carboxylic acid derivative
- Monocarboxylic acid or derivatives
- Organic oxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organooxygen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-052r-6900000000-a1ba891fd142b9683935 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-05bs-9220000000-214e5bb64f4b0e933a5d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-9f0a6e47859b044a9892 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ce9-4900000000-557fa92869acb42c653b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066u-9000000000-1b137b3fcad72dc6bfe2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-2e7b563706d62dbb82c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001r-0900000000-370071753836a7104e42 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-053i-6900000000-3f3d66fd1486eed65f3c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0900000000-864dbe09affb2fcc874f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001r-1900000000-6035878da4cadc51f802 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-2900000000-9b1bd9f17055070b5575 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-177dbd86edaaefafaba2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00kr-1900000000-75b4bbe6f9736f7f06fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0019-9700000000-8ccc7a12cc8b9757bd2f | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038181 |
|---|
| FooDB ID | FDB017420 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00010958 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 137562 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 156203 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|